Abstract
Individual differences in the locomotor response to novelty have been linked to basal differences in dopaminergic neurotransmission. Mesolimbic dopaminergic outputs are regulated by cholecystokinin (CCK), a neuropeptide implicated in anxiety. In turn, CCK expression is regulated by fibroblast growth factor-2 (FGF2), which has recently been identified as an endogenous regulator of anxiety. FGF2 binds to the high-affinity fibroblast growth factor receptor-1 (FGF-R1) to regulate the development and maintenance of dopamine neurons in the ventral tegmental area (VTA). However, the relationship between the FGF and CCK systems in the VTA is not well understood. Therefore, we utilized the selectively-bred low-responder (bLR; high-anxiety) and high-responder (bHR; low-anxiety) rats to examine the effects of repeated (21-day) FGF2 treatment on CCK and FGF-R1 mRNA in the rostral VTA (VTAr). In vehicle-treated controls, both CCK and FGF-R1 mRNA levels were increased in the VTAr of bLR rats relative to bHR rats. Following FGF2 treatment, however, bHR-bLR differences in CCK and FGF-R1 mRNA expression were eliminated, due to decreased CCK mRNA levels in the VTAr of bLR rats and increased FGF-R1 expression in bHR rats. Differences after FGF2 treatment may denote distinct interactions between the CCK and FGF systems in the VTAr of bHR vs. bLR rats. Indeed, significant correlations between CCK and FGF-R1 mRNA expression were found in bHR, but not bLR rats. Colocalization studies suggest that CCK and FGF-R1 are coexpressed in some VTAr neurons. Taken together, our findings suggest that the FGF system is poised to modulate both CCK and FGF-R1 expression in the VTAr, which may be associated with individual differences in mesolimbic pathways associated with anxietylike behavior.
Keywords: Fibroblast growth factor-2, fibroblast growth factor receptor-1, novelty response, in situ hybridization, colocalization, individual differences
Altered cholecystokinin (CCK)-mediated neurotransmission has traditionally been associated with anxiety in rodents (Chen et al., 2006; van Megen et al., 1996) and panic attacks in humans (Zwanzger et al. 2012). Unfortunately, CCK antagonism has not been effective in alleviating anxiety in clinical trials (Harro, 2006). Even so, CCK plays an important role in behavioral aspects associated with anxiety such as negative affect and stress responses (Becker et al., 2008; Benedetti et al., 2006; Harro et al., 1992; Panksepp et al., 2004). CCK is widely expressed in the brain (Beinfeld, 1983), specifically within some dopaminergic neurons located in the ventral tegmental area (VTA) (Hökfelt et al., 1980; Seroogy et al., 1989). Given that dopamine has been implicated in anxiety-like behaviors (Bertolucci-D'Angio et al., 1990; Cabib and Puglisi-Allegra, 2012; Cooper et al., 1973) the VTA is of particular interest, since this region directly modulates areas involved in the expression of reward (Crespi et al., 2000; Olson et al., 2005) and anxiety (Beiderbeck et al., 2012; de Oliveira et al., 2009; Gelowitz and Kokkinidis, 1999), including the hippocampus, amygdala, and medial prefrontal cortex (Corral-Frias et al., 2013). The neurobiological factors that regulate CCK gene expression in the VTA are not well understood. In neuroblastoma models, CCK gene transcription is regulated by fibroblast growth factor-2 (FGF2), also known as basic FGF (Hansen et al., 1999; Hansen and Nielsen, 2001). FGF2 is an important regulator of anxiety-like behavior (Turner et al., 2012), with some conflicting findings such as increased FGF2 gene expression reported in the brain of hooded PVG (anxious) rats compared to their Sprague-Dawley counterparts after exposure to the Cat-freezing test (Wang et al., 2003) and increased fear extinction following acute exogenous FGF2 administration (Graham and Richardson, 2011). To date, no study has evaluated if the CCK system interacts with the FGF system in the VTA to regulate anxiety.
Differences in locomotor response to novelty are of interest as they predict individual differences in drug self-administration (Piazza et al., 1989) and responsiveness to environmental stress (Kabbaj et al., 2000), as well as differences in the expression of CCK (Ballaz et al., 2008). Thus, distinct CCK-ergic function may contribute to promoting individual differences in the adaptation to environmental novelty (Ballaz et al., 2007). Interestingly, selectively-bred lines of high-responder (bHR; low-anxiety) and low-responder (bLR; high-anxiety) rats show differences in tyrosine hydroxylase (Clinton et al., 2012), and dopamine-mediated transmission in the mesoaccumbal system (Flagel et al., 2011) which may be associated with differences in anxiety between these two lines (Beiderbeck et al., 2012). Compared to bLRs, bHRs are more active when exposed to an inescapable, novel environment (Stead et al., 2006) and exhibit less anxiety-like behavior in tests such as the elevated plus-maze (EPM) (Perez et al., 2009). Interestingly, repeated FGF2 treatment blunts differences in anxiety-like behavior in bLR and bHR rats by reducing active-avoidance responding specifically in bLR rats, which was associated with increased neurogenesis in the hippocampus of bLR rats (Perez et al., 2009). Because hippocampal neurogenesis is, at least in part, under dopaminergic control of the VTA (Suzuki et al., 2010), and since neonatal FGF2 treatment decreased tyrosine hydroxylase in the ventral tegmental area (VTA) of bLR rats (Clinton et al., 2012), the impact of repeated FGF2 treatment on gene expression in the VTA of bLR and bHR is of interest.
In the present study, we examined the relationship between the FGF and CCK systems in the VTA. Because the actions of FGF2 in the VTA occur through binding to high-affinity tyrosine kinase receptor-1 (FGF-R1) (Guillonneau et al., 1996; Szebenyi and Fallon, 1999), FGF-R1 and CCK gene expression were examined in the rostral pole of the VTA (VTAr) of bHR and bLR rats following repeated treatment with either FGF2 or vehicle (Perez et al., 2009). We believed that the CCK system in the VTA may play an important role in the individual differences in environmental novelty-induced anxiety-like behavior that occur in response to repeated FGF2 treatment.
1. EXPERIMENTAL PROCEDURES
1.1 Subjects
Rats were obtained from our in house-breeding colony at the Molecular and Behavioral Neuroscience Institute at the University of Michigan. The bLR and bHR lines were selectively bred based on differences in locomotor activity when placed into a novel, inescapable environment (Stead et al., 2006). The gene expression data presented here correspond to a cohort of animals (Generation 19) in which novelty-driven anxiety was examined in the EPM test, a reliable index of anxiety-like behavior in rodents, following repeated FGF2 treatment, published previously by our group (Perez et al., 2009).
Briefly, the bHR-bLR phenotype was confirmed in adult male rats by assessing locomotor activity (between 9:00–11:30am) in activity boxes (43 × 21.5 × 24.5 cm) similar to the home cages placed in a different room from where rats had been housed (Stead et al., 2006). The activity boxes were flanked by photo-beam cells to track both horizontal and vertical movements of the rat which were summed to generate an overall locomotor activity score. Animals were treated in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council) and in accordance with the University of Michigan Committee on the Use and Care of Animals.
1.2 Repeated FGF-2 treatment regimen and evaluation of anxiety-like behavior
Repeated injections of either FGF2 (5ng/g, IP) or vehicle (0.1M PBS with 0.1% bovine seroalbumin) were administered once per day at 4:00pm for 21 consecutive days. On the next day (Day 22), EPM tests were conducted in a separate behavioral testing room between 8:00 and 11:30am, less than 24h following the last injection. FGF2 injections were administered to 6 bHR and 7 bLR rats, while 6 bHR and 6 bLR rats received vehicle injections.
1.3 Single-labeling in situ hybridization histochemistry
Rats were killed 24h after the conclusion of the EPM test and brains quickly removed, snap frozen in n-methylbutane (−30°C), and stored at −80°C. Brains were sectioned on a cryostat at 10µm (coronal sections), and thaw-mounted on Super Frost Plus slides (Fisher Scientific) at 100µm intervals. Tissue sections were fixed in 4% paraformaldehyde phosphate buffer for 1h, washed in 2× SSC (300mM sodium chloride, and 30mM sodium citrate buffer pH= 7.2), acetylated in 0.1M triethanolamine buffer (0.1M pH= 8.0) supplemented with acetic anhydride (0.25%) for 10 min, rinsed in distilled water, dehydrated in graded alcohol solutions (50–100%, 30s) and subsequently air-dried. Thereafter, tissues were hybridized with 35S-labeled cRNA probes.
The following probes were cloned in house: CCK, (Gene Bank access # M10353) a 260bp sequence coding for the exon III sequence of preproCCK mRNA (nucleotides 210–470) which included the sequence for the translation of the CCK octapeptide c-terminal; and FGF-R1 (Gene Bank access # NN_024146) a 657bp sequence in the coding region (nucleotides 320–977). 35S-labeled cRNA probe was diluted in 50% hybridization buffer (50% formamide, 10% dextran sulphate, 2× SSC, 50mM sodium phosphate buffer (pH= 7.4), 1× Denhardt’s solution, 0.1mg/ml yeast tRNA and 30mM dithiothreitol), 70µl of diluted probe placed on each slide (2×106 dpm per six hemi-sections/slide) and cover slipped. Slides were placed in plastic trays moistened with 50% formamide and incubated in a 55°C oven overnight. The following day, slides were rinsed in 2× SSC buffer to remove 35S-labeled probe and then incubated in RNAase A solution (200µg/ml) for 1h at 37°C. Slides were then rinsed in increasingly stringent solutions (2× SSC, 1× SSC, 0.5× SSC) and placed in 0.1× SSC at 70°C for 1h. Slides were rinsed in distilled water, dehydrated through a graded alcohol series and exposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for a period ranging from 2 days for CCK to 2 weeks for FGF-R1.
1.4 Double-label in situ hybridization histochemistry for colocalization of CCK and FGF-R1 mRNA
A subset of tissue sections was processed for dual in situ hybridization histochemistry using both radioactive (35S) and non-radioactive (digoxigenin) labeling. Non-radioactive cRNA probes complementary to either the rat CCK mRNA or FGF-R1 mRNA were labeled with digoxigenin-UTP (Dig-UTP; Boehringer Mannheim, Indianapolis) using standard transcription methods. Consecutive brain sections were hybridized with either a mixture of 35S-CCK and dig-FGF-R1 probes or with 35S-FGF-R1 and dig-CCK probes overnight at 55°C. Sections were rinsed in 2× SSC, treated with RNase A (200 µg/ml) for 1 hr at 37°C, and washed in 2×, 1×, 0.5×, and 0.1× SSC (for 5 min each). Sections were placed in 0.1× SSC at 70°C for 1h followed by immunohistochemical staining for visualization of digoxigenin-labeled probes. Brain sections were treated with a blocking solution (0.1M phosphate buffer containing 0.5% Triton X-100 and 0.25% carageenan, pH 7.5) for 4h, and then incubated overnight with an alkaline phosphatase conjugated antibody against digoxigenin (sheep anti-dig-AP, and Fab fragments, Boehringer Mannheim, Indianapolis, IN), diluted 1:20,000. After rinsing in both 0.1M phosphate buffer and 0.1M Tris buffer (30 min each), sections were incubated in the color reaction buffer containing 0.45% nitroblue tetrazolium chloride (Boehringer Mannheim), 0.35% 5-bromo-4-chloro-3-indoylphosphate 4-toluidine salt (Boehringer Mannheim), 5% polyvinyl alcohol, and 0.24% levamizole. The color reaction was completed in 3h after which sections were rinsed in water and incubated with 0.1 M glycine buffer, pH 2.2, containing 0.5% Triton X-100 for 10min. Finally, sections were fixed in 2.5% glutaraldehyde for 2h. After rinsing in water and dehydrating in a graded series of alcohols, sections were dipped in liquid emulsion (Ilford KD-5; Polysciences, Warrington, PA), air-dried, and stored in a light-tight box at 4°C for 14 days. Sections were subsequently developed, fixed, dehydrated, and coverslipped using a xylene-based mounting medium (Permount; Fisher Scientific, Houston, TX).
1.5 Image analysis of autoradiograms
Digital images were captured from the X-ray films using a CCD camera (TM-745; Pulnix, Sunnyvale, CA) and analyzed using NIH Image (Scion Beta Image 4.03) software. A template was used to standardize the shape and size of the VTA region in which the in situ hybridization signal was measured. Optical density values were corrected for background (the signal threshold was defined as the mean grey value of background plus 3.5 times the standard deviation) and then multiplied by the area of the template to yield the integrated optical density (IOD) value. IOD measures from 6–10 coronal hemi-sections per rat were averaged to generate a single value for each rat that contributed to the average IOD for each combination of treatment and phenotype. Section sampling spanned from -5.0mm to -5.6mm Bregma for VTAr according to the Paxinos and Watson atlas (2007).
1.6 Image analysis of colocalization
The microscopic cellular distribution of dual 35S-mRNA and digoxigenin hybridization signals was imaged using a Leica (Leitz, Nussloch, Germany) microscope at 400-fold magnification. Hybridization signals were visualized as a purple-blue precipitate (digoxigenin) and silver grains (35S). ImageJ (http://imagej.nih.gov/ij) software was used to count a minimum of 80 cells per field throughout 8 consecutive sections, alternately labelled for 35S-CCK/dig-FGF-R1 and 35S-FGF-R1/dig-CCK. These data points (4 for each double-labeling combination) from a single brain were then averaged.
1.7 Statistics
Statistical analyses were carried out using SPSS®16 for Windows. Differences in the expression of CCK and FGF-R1 mRNA were evaluated using a two-way ANOVA, with treatment (FGF2 vs. vehicle) and phenotype (bHR vs. bLR), and their interaction as between-subjects factors. Planned comparisons were made by using Fisher’s probable leastsquares difference (PLSD) post hoc analysis when any factor or interaction reached significance. Pearson’s correlation analyses were used to determine the magnitude of the association between FGF-R1 and CCK IOD measurements separately in bHR and bLR rats as well as in vehicle-treated and FGF2-treated rats. For all comparisons, the alpha value was set at p<0.05.
RESULTS
Differences in the expression of CCK and FGF-R1 mRNA were measured using in situ hybridization in the VTAr of bHR and bLR rats treated repeatedly with either vehicle or FGF2. Representative coronal sections through the brain (approximately −5.22mm Bregma) illustrate CCK (Fig.A1) and FGF-R1 (Fig.A2) expression in the VTAr. While there were no significant main effects of either treatment or phenotype on CCK gene expression in the VTAr, the treatment x phenotype interaction for CCK gene expression in this region reached statistical significance (F(1,19) = 5.91, P = 0.025). CCK mRNA levels in vehicle-treated bLR rats were higher than vehicle-treated bHR rats (P = 0.012), while repeated FGF2 decreased CCK mRNA levels specifically in bLR rats (vehicle-treated bLRs vs. FGF2-treated bLRs, P = 0.0048) (Fig.A3). Thus, the same repeated FGF2 treatment that has previously been shown to reduce anxiety-like behavior in bLR rats (Perez et al. 2009), also decreases CCK gene expression in the VTA.
Fig.A.
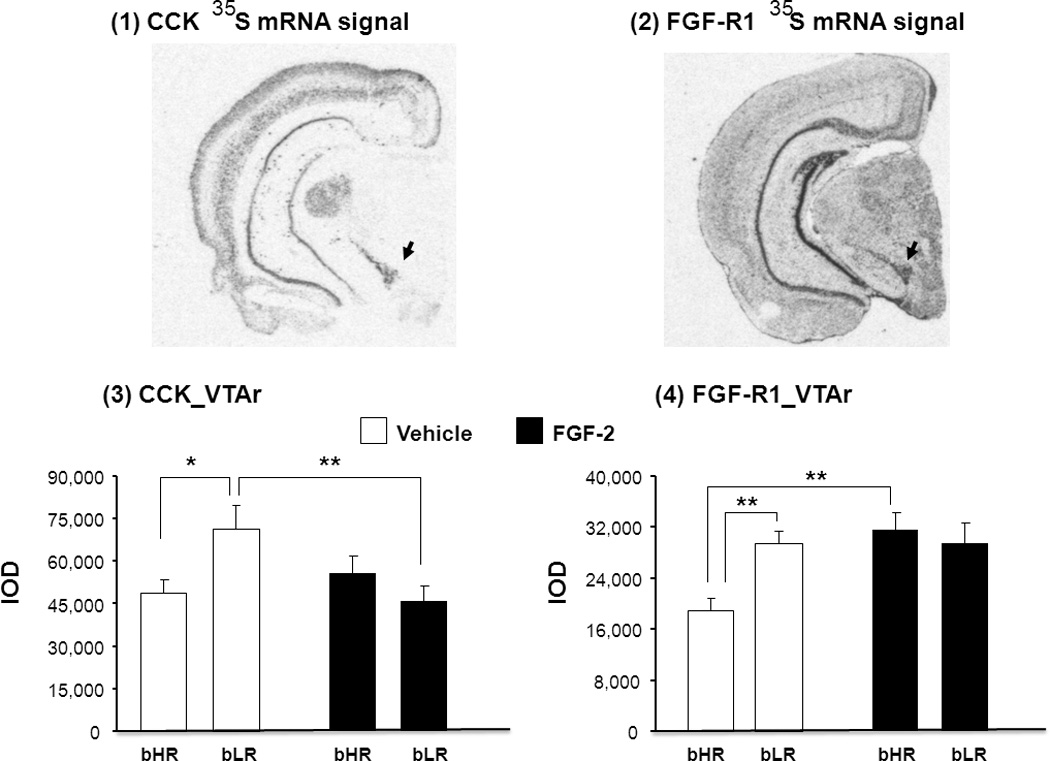
Representative autoradiograms illustrate 35S-mRNA hybridization signal for CCK (1) and FGF-R1 (2) in selected coronal hemi-sections (approx. 5.22mm posterior to Bregma) through the rostral VTA (VTAr; black arrows). The effects of repeated FGF-2 treatment on CCK (3) and FGF-R1 (4) mRNA levels are shown. Transcript levels are expressed as integrated optical density (IOD) units. Data are in mean ± S.E.M., *P<0.05, **P<0.01. N= 6–7 for each treatment group.
We then determined whether repeated FGF2 treatment altered the expression of FGF-R1, the cognate receptor for FGF2 in the VTAr. Repeated application of FGF increased FGF-R1 expression just in bHRs not in bLRs (Fig.A4; effect of treatment, F(1,24)= 6.68, P = 0.017). Although there was no main effect of phenotype, two-way ANOVA analysis revealed a significant treatment x phenotype interaction for FGF-R1 mRNA expression (F(1,24)= 6.89, P = 0.016; Fig.A4). In vehicle-treated bLRs, FGF-R1 mRNA levels were higher than vehicle-treated bHRs (P = 0.006) and repeated FGF2 treatment increased FGF-R1 gene expression specifically in bHR rats (FGF2-treated bHRs vs. vehicle-treated bHRs, (P = 0.0047). Thus, while both CCK and FGF-R1 levels were elevated in bLR rats compared to bHR rats under vehicle-treated conditions, repeated FGF2 treatment eliminated these bHR-bLR differences in the VTAr by selectively decreasing the levels of CCK in bLR rats while increasing FGF-R1 expression in bHR rats. While it could be argued that FGF-R1 mRNA did not rise in bLRs because they are already very high (ceiling effects), CCK mRNA levels in FGF2-treated bHRs were slightly higher (17%) than in vehicle-treated bHRs. Then, the lack of FGF2 effects on CCK mRNA in bHRs was not simply due to floor effects.
Because the phenotype-dependent FGF2 effects may denote different relationships between CCK and FGF system in the VTAr, we examined first the correlation between CCK and FGF-R1 in vehicle-treated and FGF2-treated rats separately, pooling both bHR and bLR rats within each treatment group. CCK mRNA signal significantly correlated with the density of the FGF-R1 signal in both the vehicle-treated group (Pearson’s correlation r = +0.524, P = 0.040; Fig.B1) and in the FGF2-treated group (Pearson’s correlation r = +0.568, P = 0.021; Fig.B2) which suggests that correlation was independent of the FGF2 treatment. Interestingly, individual differences in the relationship between CCK and FGF-R1 within each phenotype indicated that CCK and FGF-R1 mRNA expression were significantly correlated in bHR rats (Pearson’s correlation r = +0.627, P = 0.015; Fig.B3) but not in bLR rats (Pearson’s correlation r = +0.119, P = 0.349; Fig.B4). Thus, differences between locomotor response to novelty and anxiety-like behaviors between bHR and bLR lines may relate to the strength of the association between both CCK and FGF-R1 gene expression in the VTAr.
Fig.B.
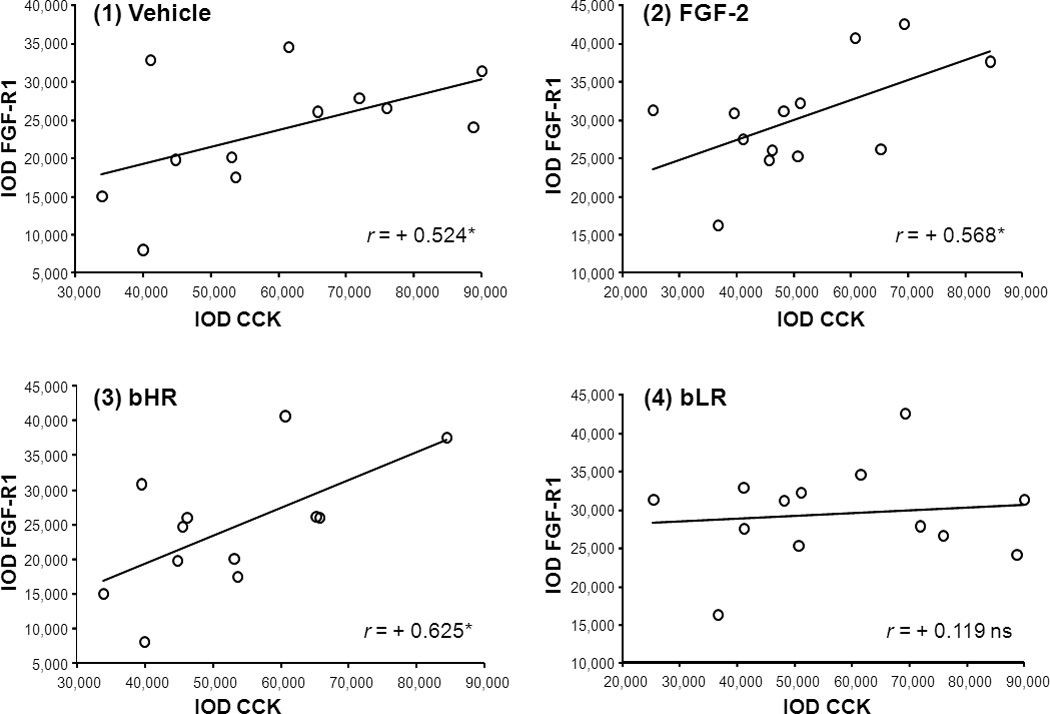
Scatter plots illustrate the relationship between CCK and FGF-R1 gene expression in (1) vehicle-treated, (2) FGF-2-treated, (3) bHR, and (4) bLR rats. For the analyses examining the correlation between CCK and FGF-R1 in vehicle (1) and FGF-2-treated (2) rats data from bHRs and bLRs were pooled. Alternatively, vehicle- and FGF-2-treated data were pooled when examining CCK and FGF-R1 correlations in bHR (3) vs bLR (4) rats. The abscissa represents CCK mRNA expression levels while the ordinate axis represents FGF-R1 mRNA levels in IOD units. The correlation index (r) denotes the strength of the association between variables. *P< 0.05 N = 12–13 (Pearson’s correlation analyses).
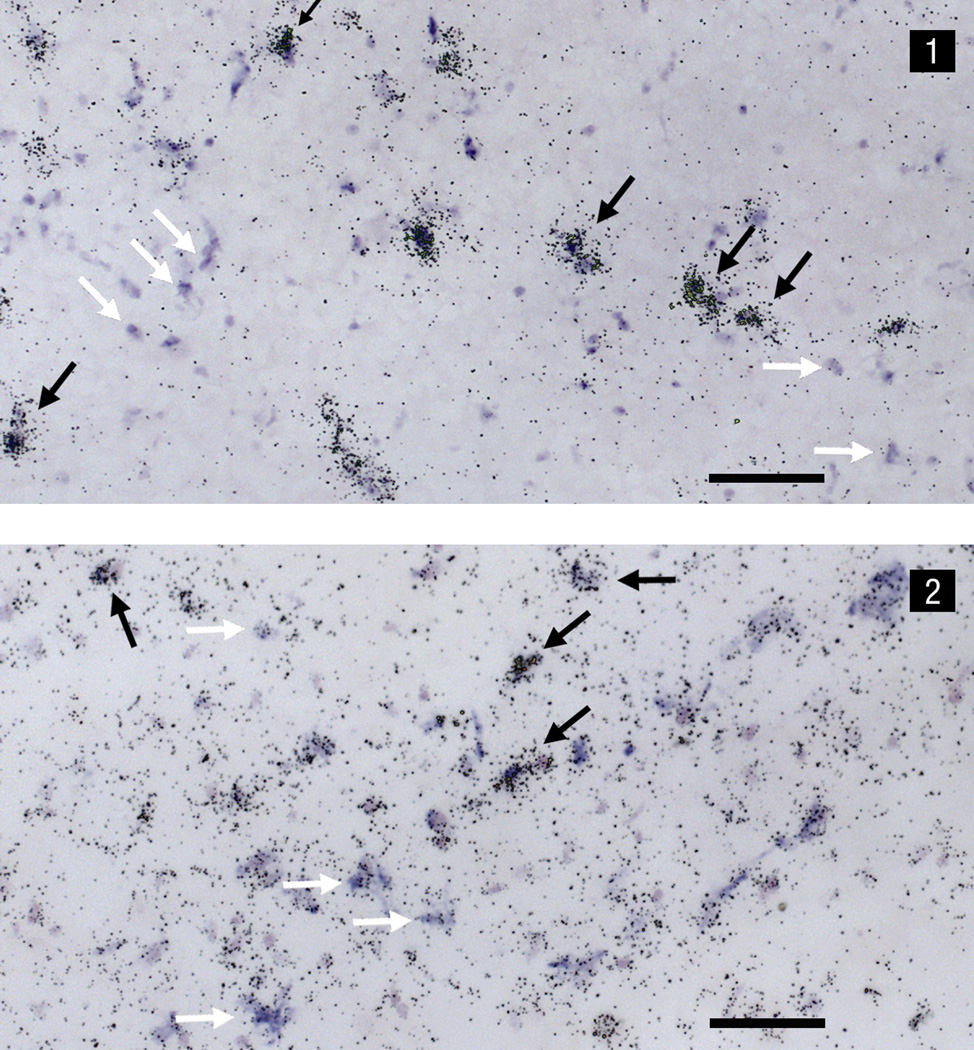
Although there is evidence for the expression of both CCK and FGF-R1 mRNA in the VTAr, it is not clear whether these mRNAs are coexpressed in individual neurons within this region. Therefore, the colocalization of CCK and FGF-R1 mRNA within individual VTAr neurons was examined in a series of 8 sections from a single control brain (4 sections each for FGF-R1-35S/CCK-Dig and FGF-R1-Dig/CCK-35S) and co-expression percentages then averaged. Dual CCK and FGF-R1 mRNA in situ hybridization histochemistry was examined using bright-field illumination (Fig.C). Approximately 17% of the total number of FGF-R1 (35S-mRNA) neurons also expressed CCK (Dig-mRNA) whilst 18% of the total number of CCK (35S-mRNA) neurons also contained FGF-R1 (Dig-mRNA), indicating that a subpopulation of neurons within the VTAr colocalize CCK and FGF-R1.
Fig.C.
Dual in situ hybridization photomicrographs under bright-field illumination of (1) FGF-R1-35S mRNA and CCK-digoxigenin mRNA, and (2) CCK-35S mRNA and FGF-R1-digoxigenin mRNA. Labeling indicating the presence of the non-radioactive digoxigenin probe appears as a blue-purple precipitate, whereas the smaller, punctate silver grains are indicative of the radioactive 35S probe. Black arrows indicate cells that express both CCK and FGF-R1 mRNA. White arrows indicate separate digoxigenin-labeled CCK mRNA (1) and 35S-labeled CCK mRNA (2) cells. Scale bar = 100µm.
DISCUSSION
Beyond their inherent differences in locomotor response to novelty, the HR and LR bred lines exhibit significant differences in coping with stress (Stead et al., 2006). Some evidence suggests the role of either FGF2 (Perez et al., 2009) or of CCK (Ballaz et al., 2007) in the adaptation to environmentally induced stress. Here, we demonstrate for the first time that a relationship between the FGF and CCK systems in the VTA may underlie individual differences in environmental novelty-driven anxiety-like behavior.
Our previous studies showed that repeated FGF2 treatment impacts anxiety-like behavior in the selectively bred low-responder (bLR) rats by decreasing anxiety-like behavior while high-responder (bHR) rats remained unaffected (Perez et al., 2009). Active-avoidance behavior, a key symptom of anxiety, has been linked to dopamine release (Copper et al., 1973). Thus, the current study examined the impact of repeated FGF2 treatment on the relationship between CCK and FGF-R1 mRNA in the rostral VTA across phenotypes. Compared to bHRs, bLRs showed increased CCK and FGF-R1 mRNA levels in the VTAr following repeated vehicle treatment. Repeated treatment with FGF2, however, eliminated these bHR-bLR differences by selectively decreasing CCK levels in bLR rats and increasing FGF-R1 expression in bHR rats. Considering the experimental approach used, we cannot assert that mRNA expression represent CCK and FGF-R1 protein levels. There is however evidence supporting a CCK-FGF-R1 relationship. The expression of CCK and FGF-R1 gene expression was correlated in bHRs, but not bLRs, suggesting that their differences in anxiety-like behaviors may relate to the strength of the association between the FGF and CCK systems, which is supported by evidence indicating that CCK and FGF-R1 are colocalized in a subset of VTAr neurons.
The locomotor response to a novel environment may be associated with dysregulation of a complex neurotransmitter network (Flagel et al., 2011; Clinton et al., 2012), including alterations in CCK within discrete areas of the VTA (i.e., rostral pole). The present study suggests that CCK gene expression in bLR rats was higher than in bHR rats in the VTAr, consistent with our previous work in outbred rats classified as high- or low-responders (Ballaz et al., 2008). This reveals that the direction of alterations of basal CCK mRNA levels were not due to the breeding selection, but to the genetic endowment. There is evidence to suggest that higher CCK levels in the VTA may be associated with the increased level of anxiety-like behaviour in bLR rats (Hebb et al., 2002). Indeed, here we show that CCK gene expression was decreased in the VTAr of bLR rats following FGF2 treatment (Fig.C1), the same bLR rats in which the anxiolytic-like effects of repeated FGF2 were reported (Perez et al. 2009). While decreased anxiety-like behavior in FGF2-treated bLR rats was attributed to changes in hippocampal neurogenesis (Perez et al., 2009), it should be noted that cell proliferation in the subgranular zone of the dentate gyrus is also likely mediated by dopaminergic neurons located in the VTA (Suzuki et al., 2010). There is evidence that administration of anxiogenic drugs results in an increase in CCK mRNA levels in several brain regions known to be involved in the regulation of anxiety (Pratt & Brett, 1995; Sherrin et al., 2009) and the VTA is an important component of the anxiety neurocircuitry (Beiderbeck et al., 2012; Corral-Frias et al., 2013; de Oliveira et al., 2009; Gelowitz & Kokkinidis, 1999) where the provocation of anxiety-like behavior is associated with increased mesocorticolimbic CCK mRNA (Hebb et al., 2002). Thus, the FGF2-induced reduction of CCK gene expression in the VTA of bLR rats, presumably via actions at FGF-R1 (Klejbor et al., 2006), may also account for its behavioral effects in the EPM.
FGF2-induced changes in CCK and FGF-R1 transcripts were detected in the rostral pole of the VTA, suggesting a linkage between FGF2 signaling and CCK gene expression which may be determinant in the function of the mesolimbic system across phenotypes (see figures B and C). There is evidence that FGF2 contributes to psychostimulant sensitization (Flores et al., 2010, Clinton et al., 2012), a role shared by endogenous CCK (Beinfeld, 2003). Given the positive correlation between FGF-R1 and CCK transcript levels observed in the VTA of bHR rats, the increase of FGF-R1 gene expression is expected to influence their vulnerability to psychostimulants (Turner et al., 2008a). Although it is possible that other FGF family members (e.g., FGF1) may be acting at the high-affinity receptor FGF-R1 (Coutts and Gallagher, 1995), FGF-R1 function in midbrain dopaminergic neurons specifically relies on the FGF2 neurotransmission (Beck, 1994; Gonzalez et al., 1995; Klejbor et al., 2006; Ratzka et al., 2012). Moreover, we present evidence indicating that FGF-R1 and CCK are colocalized in some VTAr neurons (Fig.C), supporting the direct actions of FGF2 treatment on CCK neurons, and potentially dopamine, in this region. The VTAr is populated by both GABAergic and dopaminergic neurons (Olson and Nestler, 2007) and there is evidence that CCK colocalizes with GAD, a marker of GABAergic cells (Olson and Nestler, 2007), as well as tyrosine hydroxylase, the rate limiting enzyme in the production of dopamine, in this region (Hökfelt et al., 1980). That significant correlations between CCK and FGF-R1 mRNA expression were found in bHR, but not bLR rats suggests that FGF2 actions at FGF-R1 impact multiple neuronal populations in the VTAr through a mechanism involving CCK. This anatomical relationship warrants further research to examine individual differences in the CCK-FGF-R1 link to anxiety-like behavior.
The current study presents the first evidence for an interaction between CCK and FGF2 in vivo, as previous reports of an interaction have only been described in cell-based in vitro models. CCK and FGF2 had additive trophic effects in primary cultures of rat pancreatic acinar cells (Hoshi and Logsdon, 1993) and intracellular signalling studies in both neuroblastoma (Hansen et al., 1999) and enteroendocrin (Ratineau et al., 2001) cell lines demonstrated that FGF2 regulated the CCK gene expression. In this vein, a significant correlation between CCK and FGF-R1 mRNA expression was found in bHR rats. Our current data also suggest that repeated FGF2 can impact the expression of CCK in vivo in bLR rats, presumably through the FGF-R1 receptor with behavioural consequences. There is evidence that peripherally administered (IP) FGF2 crosses the blood–brain barrier and rapidly enters into the brain (Cuevas et al., 1996; Deguchi et al., 2000) where it can induce emotional changes (Turner et al., 2008b). The effects of chronic, peripheral administration of FGF2 presented here can be attributed to its direct pharmacological action on the brain (Perez et al., 2009; Wagner et al., 1999). In light of our data, we cannot explain the exact mechanisms underlying FGF-induced reduction of CCK expression in bLRs as experiments were performed by exogenous application of FGF2. Testing the role of endogenous FGF2 in modulating CCK expression would undoubtedly demonstrate the exact relationship of these two systems.
Conclusions
In sum, repeated treatment with FGF2, which acts as an anxiolytic in bLR rats, eliminated bHR-bLR differences in the expression of CCK and FGF-R1 mRNA levels in the VTAr. This denotes an interaction of the CCK and FGF systems, as demonstrated by the different level of CCK-FGF-R1 mRNA correlation across phenotypes, in shaping individual differences in novelty-induced anxiety that awaits further research.
Highlights.
FGF2 blunts the anxiety gap between bred low- and high-responder (bHR/bLR) rats
CCK and FGF-R1 mRNA levels were increased in the VTA of bLRs relative to bHRs
Repeated FGF2 lowered CCK and rose FGF-R1 mRNAs in bLRs and bHRs respectively
CCK and FGF-R1 mRNA expression in the VTA correlates in bHRs, but does not in bLRs
CCK and FGF-R1 are coexpressed in the VTA and may be related to individual anxiety
ACKNOWLEDGMENTS
We are extremely grateful to Jennifer Fitzpatrick and Sharon Burke for their technical assistance. Selectively bred HR and LR rats were generously provided by Sarah Clinton.
This work was supported by NIDA P01 DA021633, The Office of Naval Research (ONR) Grants N00014-09-1-0598 and N00014-12-1-0366 to SJW and HA, NIMH Grant 20030 to JP, as well as the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (http://www.pritzkerneuropsych.org).
ABBREVIATIONS
- bHR
bred high-responders
- bLR
bred low-responders
- CCK
cholecystokinin
- Dig
digoxigenin
- FGF-R1
fibroblast growth factor receptor-1
- FGF2
fibroblast growth factor-2 (basic fibroblast growth factor)
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests.
Contributor Information
Santiago J Ballaz, Email: sballazg@gmail.com.
Javier Perez, Email: perezja12@gmail.com.
Maria Waselus, Email: mwaselus@umich.edu.
Huda Akil, Email: akil@umich.edu.
Stanley J Watson, Email: watsons@umich.edu.
REFERENCES
- Ballaz S, Akil H, Watson SJ. The CCK-system mediates adaptation to novelty-induced stress in the rat: a pharmacological evidence. Neurosci Lett. 2007;428:27–32. doi: 10.1016/j.neulet.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Ballaz S, Akil H, Watson SJ. The CCK-system underpins novelty-seeking behavior in the rat: gene expression and pharmacological analysis. Neuropeptides. 2008;42:245–253. doi: 10.1016/j.npep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD. Functions of brain-derived growth factor, insuline-like growth factor-1 and basic fibroblast growth factor in the development and maintenance of dopaminergic neurons. Prog Neurobiol. 1994;44:497–516. doi: 10.1016/0301-0082(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety -Involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37:1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983;3:411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. What we know and what we need to know about the role of endogenous CCK in psychostimulant sensitization. Life Sci. 2003;73:643–654. doi: 10.1016/s0024-3205(03)00384-9. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolucci-D'Angio M, Serrano A, Scatton B. Mesocorticolimbic dopaminergic systems and emotional states. J Neurosci Methods. 1990;34:135–142. doi: 10.1016/0165-0270(90)90051-g. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Chen Q, Nakajima A, Meacham C, Tang YP. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc Natl Acad Sci USA. 2006;103:3881–3886. doi: 10.1073/pnas.0505407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Turner CA, Flagel SB, Simpson DN, Watson SJ, Akil H. Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacol Biochem Behav. 2012;103:6–17. doi: 10.1016/j.pbb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BR, Breese GR, Grant LD, Howard JL. Effects of 6-hydroxydopamine treatments on active avoidance responding: evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973;185:358–370. [PubMed] [Google Scholar]
- Coutts JC, Gallagher JT. Receptors for fibroblast growth factors. Immunol Cell Biol. 1995;73:584–589. doi: 10.1038/icb.1995.92. [DOI] [PubMed] [Google Scholar]
- Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology. 2013;38:350–363. doi: 10.1038/npp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi F, Corsi M, Regianni A, Ratti E, Gaviraqhi C. Involvement of cholecystokinin within craving for cocaine: role of cholecystokinin receptor ligands. Expert Opin Investig Drugs. 2000;9:2249–2258. doi: 10.1517/13543784.9.10.2249. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Fernández-Ayerdi A, Carceller F, Colin S, Mascarelli F, Muñoz-Willery I, Giménez-Gallego G. Central nervous system distribution of fibroblast growth factor injected into the blood stream. Neurol Res. 1996;18:267–272. doi: 10.1080/01616412.1996.11740418. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Naito T, Yuge T, Furukawa A, Yamada S, Pardridge WM, Kimura R. Blood-brain barrier transport of 125I-labeled basic fibroblast growth factor. Pharm Res. 2000;17:63–69. doi: 10.1023/a:1007570509232. [DOI] [PubMed] [Google Scholar]
- de Oliveira AR, Reimer AE, Brandão ML. Role of dopamine receptors in the ventral tegmental area in conditioned fear. Behav Brain Res. 2009;199:271–277. doi: 10.1016/j.bbr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores JA, Galan-Rodriguez B, Rojo AI, Ramiro-Fuentes S, Cuadrado A, Fernandez-Espejo E. Fibroblast growth factor-1 within the ventral tegmental area participates in motor sensitizing effects of morphine. Neuroscience. 2010;165:198–211. doi: 10.1016/j.neuroscience.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Gelowitz DL, Kokkinidis L. Enhanced amygdala kindling after electrical stimulation of the ventral tegmental area: implications for fear and anxiety. J Neurosci. 1999;19:RC41. doi: 10.1523/JNEUROSCI.19-22-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau X, Tassin J, Berrou E, Bryckaert M, Courtois Y, Mascarelli F. In vitro changes in plasma membrane heparan sulfate proteoglycans and in perlecan expression participate in the regulation of fibroblast growth factor 2 mitogenic activity. J Cell Physiol. 1996;166:170–187. doi: 10.1002/(SICI)1097-4652(199601)166:1<170::AID-JCP19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Memory of fearful events: the role of fibroblast growth factor-2 in fear acquisition and extinction. Neuroscience. 2011;189:156–169. doi: 10.1016/j.neuroscience.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Rehfeld JF, Nielsen FC. Mitogen-activated protein kinase and protein kinase A signaling pathways stimulate cholecystokinin transcription via activation of cyclic adenosine 3´,5´-monophosphate response element-binding protein. Mol Endocrinol. 1999;13:466–475. doi: 10.1210/mend.13.3.0257. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Nielsen FC. Regulation of neuronal cholecystokinin gene transcription. Scand J Clin Lab Invest. 2001;234(Suppl):61–67. [PubMed] [Google Scholar]
- Harro J. CCK and NPY as anti-anxiety treatment targets: promises, pitfalls, and strategies. Amino Acids. 2006;31:215–230. doi: 10.1007/s00726-006-0334-x. [DOI] [PubMed] [Google Scholar]
- Harro J, Marcusson J, Oreland L. Alterations in brain cholecystokinin receptors in suicide victims. Eur Neuropsychopharmacol. 1992;2:57–63. doi: 10.1016/0924-977x(92)90037-9. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Dominguez H, Trudel F, Laforest S, Drolet G. Odor-induced variation in anxiety-like behavior in mice is associated with discrete and differential effects on mesocorticolimbic cholecystokinin mRNA expression. Neuropsychopharmacology. 2002;27:744–755. doi: 10.1016/S0893-133X(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Rehfeld JF, Skirbol LL, Ivermark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurons. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Logsdon CD. Direct trophic effects of fibroblast growth factors on rat pancreatic acinar cells in vitro. Biochem Biophys Res Commun. 1993;196:1202–1207. doi: 10.1006/bbrc.1993.2379. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klejbor I, Myers JM, Hausknecht K, Corso TD, Gambino AS, Morys J, Maher PA, Hard R, Richards J, Stachowiak EK, Stachowiak MK. Fibroblast growth factor receptor signalling affects development and function of dopamine neurons - inhibition results in a schizophrenia-like syndrome in transgenic mice. J Neurochem. 2006;97:1243–1258. doi: 10.1111/j.1471-4159.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, Moskal JR. Regional brain cholecystokinin changes as a function of friendly and aggressive social interactions in the rat. Brain Res. 2004;1025:75–84. doi: 10.1016/j.brainres.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotactic coordinates Ed 6. London: Academic Press; 2007. [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administrtation. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Perez J, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF-2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JA, Brett RR. The benzodiazepine receptor inverse agonist FG 7142 induces cholecystokinin gene expression in rat brain. Neurosci Lett. 1995;184:197–200. doi: 10.1016/0304-3940(94)11205-w. [DOI] [PubMed] [Google Scholar]
- Ratineau C, Dreau S, Blanc M, Bernard C, Cordier-Bussat M, Abello J, Chayvialle J, Roche C. CCK expression in enteroendocrine cell is regulated by soluble factor(s) from underlying fibroblasts. Mol Cell Endocrinol. 2001;175:5–13. doi: 10.1016/s0303-7207(01)00431-2. [DOI] [PubMed] [Google Scholar]
- Ratzka A, Baron O, Stachowiak MK, Grothe C. Fibroblast growth factor 2 regulates dopaminergic neuron development in vivo. J Neurochem. 2012;122:94–105. doi: 10.1111/j.1471-4159.2012.07768.x. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Dangaran K, Lim S, Haycock JW, Fallon JH. Ventral mesencephalic neurons containing both cholecystokinin- and tyrosine hydroxylase-like immunoreactivities project to forebrain regions. J Comp Neurol. 1989;279:397–414. doi: 10.1002/cne.902790306. [DOI] [PubMed] [Google Scholar]
- Sherrin T, Todorovic C, Zeyda T, Tan CH, Wong PT, Zhu YZ, Spiess J. Chronic stimulation of corticotropin-releasing factor receptor 1 enhances the anxiogenic response of the cholecystokinin system. Mol Psychiatry. 2009;14:291–307. doi: 10.1038/sj.mp.4002121. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviours. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, Takahashi T, Suda S, Matsuzaki H, Iwata Y, Hashimoto K, Mori N. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS One. 2010;5:e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signalling factors. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett. 2008a;446:105–107. doi: 10.1016/j.neulet.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008b;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron. 2012;76:160–174. doi: 10.1016/j.neuron.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Megen HJ, Westenberg HG, den Boer JA, Kahn RS. Cholecystokinin in anxiety. Eur Neuropsychopharmacol. 1996;6:263–280. doi: 10.1016/s0924-977x(96)00038-7. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhu YZ, Wong PT, Farook JM, Teo AL, Lee LK, Moochhala S. cDNA microarray analysis of gene expression in anxious PVG and SD rats after cat-freezing test. Exp Brain Res. 2003;149:413–421. doi: 10.1007/s00221-002-1369-1. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Domschke K, Bradwejn J. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress Anxiety. 2012;29:762–774. doi: 10.1002/da.21919. [DOI] [PubMed] [Google Scholar]