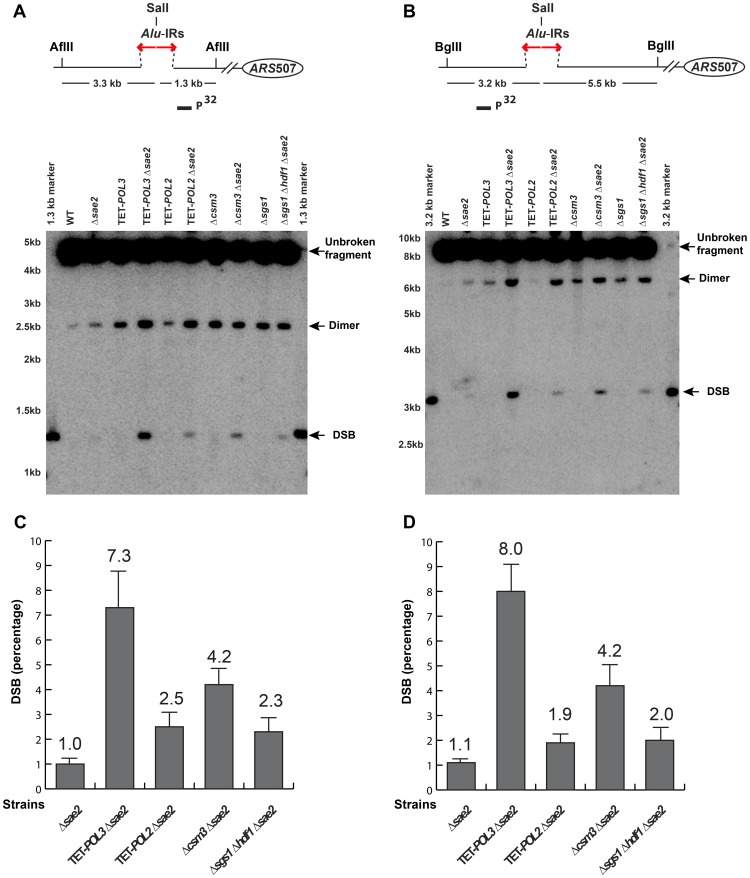
Figure 2. Physical detection of breakage intermediates in the wild-type and mutant strains carrying 100% Alu-IRs.
Yeast genomic DNA embedded in agarose plugs was digested with either AflII (A) or BglII (B). The relative positions of the repeats, the restriction sites and the replication origin ARS507 are illustrated. Digested DNA was separated by gel electrophoresis. Southern hybridization using LYS2-specific probes (solid rectangles) was carried out to detect the chromosomal fragments centromere-proximal (A) or distal (B) to the breaks induced by Alu-IRs. For the centromere-proximal intermediates (A), the size of unbroken fragment, dimer and DSB fragment are 4.6 kb, 2.6 kb and 1.3 kb, respectively. For the centromere-distal intermediates (B), the size of unbroken fragment, dimer and DSB fragment are 8.7 kb, 6.4 kb and 3.2 kb, respectively. Bands corresponding to the unbroken fragment, dimer and DSB fragment are indicated by arrows. The 1.3 kb marker and 3.2 kb marker were generated by digesting genomic DNA from the wild-type Alu-IRs strain with SalI+AflII (A) or SalI+BglII (B), where SalI cuts inside the 12 bp spacer of the Alu-IRs. The strains used for analysis are: wild-type, Δsae2, TET-POL3, TET-POL3Δsae2, TET-POL2, TET-POL2Δsae2, Δcsm3, Δcsm3Δsae2, Δsgs1, Δsgs1Δhdf1Δsae2. (C) and (D) Densitometry analysis of the broken fragments normalized to the intact chromosome V in Δsae2 strains in (A) and (B), respectively. Values are shown as mean (shown on the top of the bars) with standard deviation obtained from at least three independent experiments.