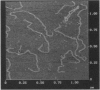
Abstract
Direct imaging with the atomic force microscope has been used to identify specific nucleotide sequences in plasmid DNA molecules. This was accomplished using EcoRI (Gln-111), a mutant of the restriction enzyme that has a 1000-fold greater binding affinity than the wild-type enzyme but with cleavage rate constants reduced by a factor of 10(4). ScaI-linearized plasmids with single (pBS+) and double (pGEM-luc and pSV-beta-galactosidase) EcoRI recognition sites were imaged, and the bound enzyme was localized to a 50- to 100-nt resolution. The high affinity for the EcoRI binding site exhibited by this mutant endonuclease, coupled with an observed low level of nonspecific binding, should prove valuable for physically mapping large DNA clones by direct atomic force microscope imaging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian W. J., Spraker T. R., Davies R. B. Epornitics of aspergillosis in mallards (Anas platyrhynchos) in north central Colorado. J Wildl Dis. 1978 Apr;14(2):212–217. doi: 10.7589/0090-3558-14.2.212. [DOI] [PubMed] [Google Scholar]
- Bezanilla M., Drake B., Nudler E., Kashlev M., Hansma P. K., Hansma H. G. Motion and enzymatic degradation of DNA in the atomic force microscope. Biophys J. 1994 Dec;67(6):2454–2459. doi: 10.1016/S0006-3495(94)80733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomley M. J., Nicholson D. A., Bartal G., Bradley A., Myers M., Allison D. J. Penetration of the holmium:YAG laser through fluid. J Vasc Interv Radiol. 1995 Nov-Dec;6(6):903–910. doi: 10.1016/s1051-0443(95)71210-6. [DOI] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- Erie D. A., Yang G., Schultz H. C., Bustamante C. DNA bending by Cro protein in specific and nonspecific complexes: implications for protein site recognition and specificity. Science. 1994 Dec 2;266(5190):1562–1566. doi: 10.1126/science.7985026. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Gupta M., Boyer H. W., Brown W. E., Rosenberg J. M. Sequence analysis of the DNA encoding the Eco RI endonuclease and methylase. J Biol Chem. 1981 Mar 10;256(5):2143–2153. [PubMed] [Google Scholar]
- Griffith J. D. Electron microscopic visualization of DNA in association with cellular components. Methods Cell Biol. 1973;7:129–146. doi: 10.1016/s0091-679x(08)61774-4. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Bezanilla M., Zenhausern F., Adrian M., Sinsheimer R. L. Atomic force microscopy of DNA in aqueous solutions. Nucleic Acids Res. 1993 Feb 11;21(3):505–512. doi: 10.1093/nar/21.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. Imaging and nanodissection of individual supercoiled plasmids by atomic force microscopy. Nucleic Acids Res. 1992 Feb 11;20(3):445–447. doi: 10.1093/nar/20.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClarin J. A., Frederick C. A., Wang B. C., Greene P., Boyer H. W., Grable J., Rosenberg J. M. Structure of the DNA-Eco RI endonuclease recognition complex at 3 A resolution. Science. 1986 Dec 19;234(4783):1526–1541. doi: 10.1126/science.3024321. [DOI] [PubMed] [Google Scholar]
- Newman A. K., Rubin R. A., Kim S. H., Modrich P. DNA sequences of structural genes for Eco RI DNA restriction and modification enzymes. J Biol Chem. 1981 Mar 10;256(5):2131–2139. [PubMed] [Google Scholar]
- Pietrasanta L. I., Schaper A., Jovin T. M. Probing specific molecular conformations with the scanning force microscope. Complexes of plasmid DNA and anti-Z-DNA antibodies. Nucleic Acids Res. 1994 Aug 25;22(16):3288–3292. doi: 10.1093/nar/22.16.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry B. J., Jack W. E., Modrich P. Facilitated diffusion during catalysis by EcoRI endonuclease. Nonspecific interactions in EcoRI catalysis. J Biol Chem. 1985 Oct 25;260(24):13130–13137. [PubMed] [Google Scholar]
- Thundat T., Allison D. P., Warmack R. J., Brown G. M., Jacobson K. B., Schrick J. J., Ferrell T. L. Atomic force microscopy of DNA on mica and chemically modified mica. Scanning Microsc. 1992 Dec;6(4):911–918. [PubMed] [Google Scholar]
- Thundat T., Allison D. P., Warmack R. J., Ferrell T. L. Imaging isolated strands of DNA molecules by atomic force microscopy. Ultramicroscopy. 1992 Jul;42-44(Pt B):1101–1106. doi: 10.1016/0304-3991(92)90409-d. [DOI] [PubMed] [Google Scholar]
- Vesenka J., Guthold M., Tang C. L., Keller D., Delaine E., Bustamante C. Substrate preparation for reliable imaging of DNA molecules with the scanning force microscope. Ultramicroscopy. 1992 Jul;42-44(Pt B):1243–1249. doi: 10.1016/0304-3991(92)90430-r. [DOI] [PubMed] [Google Scholar]
- Wright D. J., King K., Modrich P. The negative charge of Glu-111 is required to activate the cleavage center of EcoRI endonuclease. J Biol Chem. 1989 Jul 15;264(20):11816–11821. [PubMed] [Google Scholar]