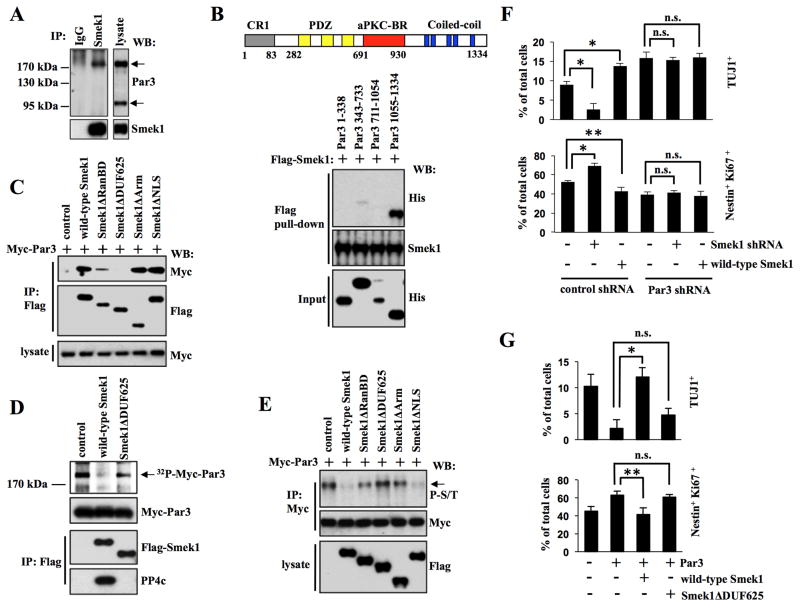
Figure 3. Smek1 interacts with Par3 and inhibits its function in neuronal differentiation.
(A) Smek1 binds to Par3. NPC lysates were immunoprecipitated with IgG or Smek1 antibodies, and subjected to Western blotting with indicated antibodies.
(B) Smek1 recognizes the Par3 coiled-coil region (amino acids 1055–1334). Upper, schematic structure of Par3 structure containing CR1 (gray, conserved region 1), PDZ (yellow, PSD-95/Dgl/ZO-1), aPKC-BR (red, aPKC-binding region), and coiled-coil (blue, Coiled-coil region) domains. Lower, Flag-Smek1 protein-immobilized beads were incubated with purified His-tagged Par3 protein fragments, and bound proteins were analyzed by Western blotting using anti-His or -Flag antibodies.
(C) The Smek1 DUF625 domain is required for Par3 binding. NPCs were transduced with lentiviruses expressing wild-type Smek1 or Smek1 mutants together with lentivirus expressing Myc-Par3. Anti-Flag immunoprecipitates were analyzed by Western blotting using anti-Myc and -Flag antibodies.
(D) Par3 is dephosphorylated by the Smek1/PP4c complex. 293T cells transfected with wild-type Flag-Smek1, Flag-Smek1ΔDUF625, or a control construct. The Smek1/PP4c-containing protein complex was isolated by anti-Flag immunoprecipiation. Myc-Par3 was 32P-labeled using an NPS lysate and purified by anti-Myc immunoprecipitation. 32P-labeled Myc-tagged Par3 was subjected to a dephosphorylation assay in the presence of a Smek1/PP4c complex. Anti-Flag immunoprecipitates were also subjected to Western blotting with anti-Myc, -Flag, and -PP4c antibodies.
(E) Western blot analysis of anti-Myc immunoprecipitates from NPCs described in C. Western blotting with an anti-phospho-serine/threonine antibody shows that both wild-type Smek1 and the Smek1ΔNLS mutant decrease Par3 phosphorylation.
(F) Smek1 acts upstream of Par3 in neurogenesis. NPCs expressing Smek1 shRNA, wild-type Smek1, Par3 shRNA, or control shRNA were cultured for 1 day under differentiating conditions and then immunostained with TUJ1 or Nestin plus Ki67 antibodies. Percentages of neurons or undifferentiated NPCs were determined by comparing TUJ1-positive or Nestin/Ki67 double-positive cells to total cells, respectively. Error bars represent the mean ± S.D. of three independent experiments. *p < 0.001; **p < 0.05; n.s., not significant.
(G) Smek1 suppresses Par3 regulation of neuronal differentiation. NPCs transduced with lentivirus expressing Par3, wild-type Smek1, Smek1ΔDUF625 mutant, or a control construct were cultured for 1 day under differentiating conditions and then immunostained with TUJ1 or Nestin plus Ki67 antibodies. Percentages of neurons and NPCs were evaluated as described in F. *p < 0.001; **p < 0.05; n.s., not significant.