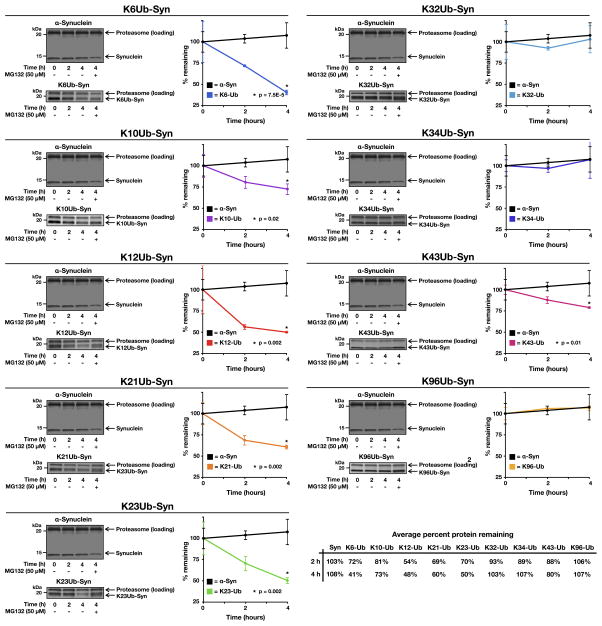
Figure 3. see also Figure S3. Proteasome-dependent degradation of monoubiquitinated α-synuclein.
Unmodified or monoubiquitinated α-synuclein proteins were incubated in triplicate with 26S proteasome for the indicated lengths of time. The proteasome inhibitor MG132 (100 μM) was added as indicated. The proteins were separated by SDS-PAGE, stained with colloidal silver, and quantitated using Quantity One Analysis Software (Bio-Rad). The turnover of unmodified α-synuclein is shown in each panel for comparison. P, two-tailed t-test; error bars represent ±s.e.m.