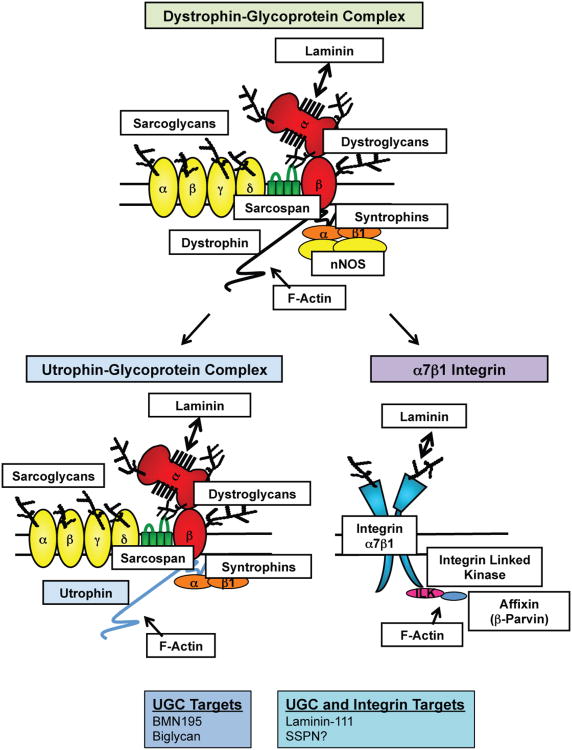
Figure 1. UGC- and α7β1 integrin-mediated replacement therapy for the DGC in DMD.
The DGC, UGC, and α7β1 integrins function to prevent contraction-induced damage of the sarcolemma by maintaining connections between the actin cytoskeleton and ECM. The DGC is composed of dystrophin, the dystroglycans (α- and β-DG), the sarcoglycans (α-, β-, γ-, and δ-SG), sarcospan (SSPN), and the syntrophins (α- and β-subunits). Neuronal nitric oxide synthase (nNOS) requires dystrophin and syntrophin to be anchored to the sarcolemmal membrane, where it is thought to function in preventing functional muscle ischemia. The UGC is homologous to the DGC, where utrophin replaces dystrophin. However, many differences exist between the UGC and DGC, including the glycosylation of α-DG, the domains in which actin binding occurs, and the lack of nNOS binding sites on utrophin. α7β1 integrin differs from the UGC/DGC in the globular domains by which laminin binds and the presence of adaptor proteins that facilitate actin binding, including ILK and β-parvin. BMN195 and biglycan are two utrophin upregulation therapeutics that are near/in clinical trials and laminin 111 is the only dual (utrophin and integrin) target therapy that is near clinical trials. This review proposes that AAV delivery of SSPN should be considered as an additional dual target therapy.