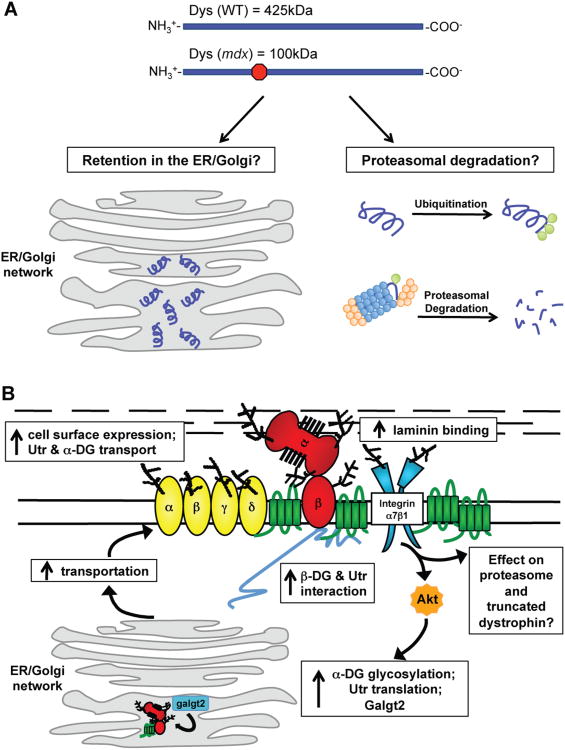
Figure 3. Effects of SSPN over-expression in mdx mice on the cell surface protein expression and protein processing and possible outcomes of truncated dystrophin within the cell.
(A) Wild-type mice express full-length dystrophin, which is 425 kDa. The mdx mutation leads to a premature stop codon that results in a truncated dystrophin of 100 kDa. Dystrophin is depicted in purple, the proteasome is blue and orange, and ubiquitin is green. Truncated dystrophin can be retained in the ER/Golgi. Alternatively, truncated dystrophin is ubiquitinated and sent to the proteasomal degradation pathway. (B) The over-expression of SSPN in mdx muscle leads to molecular events resulting in the restoration of laminin-binding and rescue of mdx pathology. SSPN activates Akt, which leads to an increase in utrophin and integrins. Galgt2, one enzyme responsible for GalNAc modification of α-DG, is also increased in isolated ER/Golgi membranes. SSPN also improves utrophin-DG transportation to the sarcolemma while simultaneously restoring laminin-binding and membrane stability. SSPN's effect on the trafficking of truncated dystrophin and proteasomal degradation is still unknown. DGs (red), SGs (yellow), SSPN (green), integrins (blue), and Akt (orange) are shown. Utrophin (Utr) is depicted in gray.