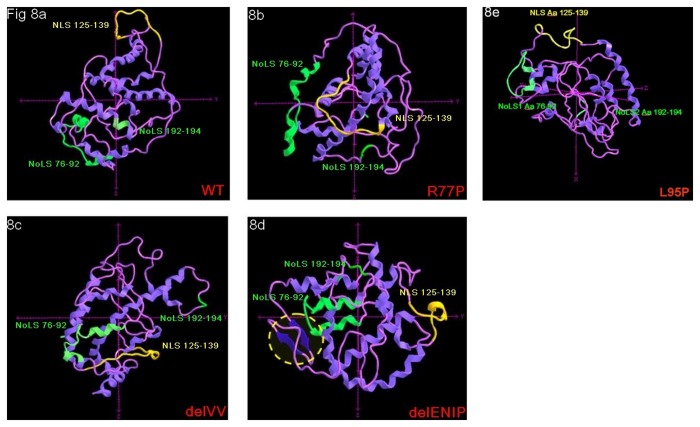
Figure 8. 3-D modelling prediction.
The different localization and folding level of the NLS 125-139, NoLS 76-92 and NoLS 192-194 are shown; a: WT protein, the NLS 125-139 and the NoLS 76-92 locate at the outer of the protein; b and c: for the R77P and delVV mutant proteins, while the NoLS 76-92 do not show steric hindrance, the NLS 125-139 appears located within the protein structure; d: in case of the delENIP mutant the NLS 125-139 appeared strongly misfolded while two novel antiparallel β sheet fragments (Aa 15-27) are shaped and seem to contribute to the general inaccessibility of the NLS; e: the L95P mutation seems not to alter the either the conformation nor the accessibility of the NLS, as for the WT sequence: note that only for this model, axys are differently orientated to facilitate the observation of the protein motifs.