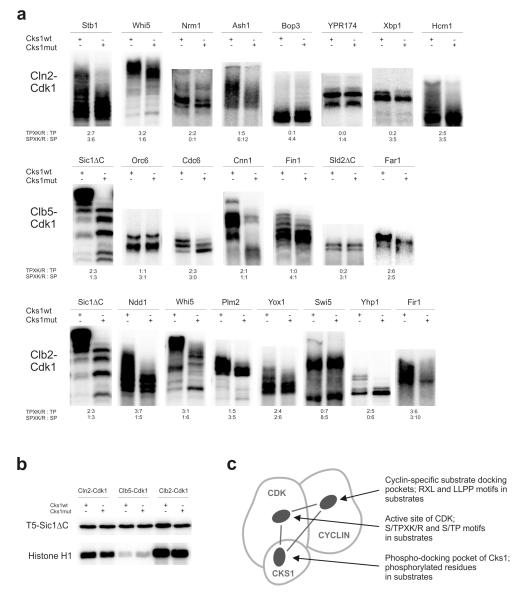
Figure 1. Cks1-dependent multisite phosphorylation of Cdk1 targets.
(a) Demonstration of Cks1-dependent accumulation of multi-phosphorylated forms in selected targets of Cdk1. The kinase assays were performed using purified Cln2-, Clb5-, and Clb2-Cdk1 complexes, which were preincubated with either wild-type Cks1 or Cks1mut (phospho-pocket mutant). The radioactively labeled multi-phosphorylated forms were separated using Phos-Tag SDS PAGE. The number of optimal and suboptimal consensus motifs, together with an indication of whether the sites have serine or threonine residue as the phospho-acceptor, are provided below the panels; (b) Phosphorylation of a substrate construct containing a single CDK consensus phosphorylation site (T5-Sic1ΔC) is not influenced by Cks1-dependent phospho-docking. Additionally, the phosphorylation assays using a standard substrate histone H1 are shown; (c) A schematic diagram indicating three pockets in cyclin-Cdk1-Cks1 complex whose local site-specificity and positioning geometry could potentially control the phosphorylation dynamics of multisite targets. The uncropped autoradiography scans are provided in Supplementary Figure 8.