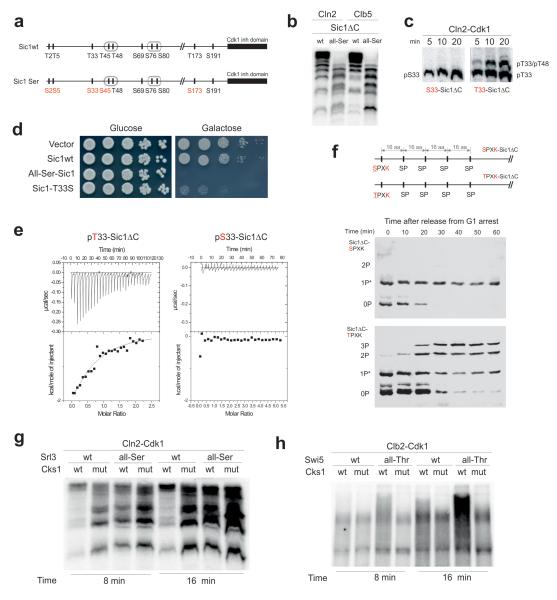
Figure 2. Exclusive preference of threonine over serine residues as the priming sites for Cks1-dependent docking and phosphorylation steps.
(a) A diagram indicating the Thr-to-Ser substitutions made in Sic1 for the experiments in panels ‘b’ and ‘d’; (b) The kinase assay demonstrating the loss of abrupt multisite phosphorylation caused by Thr-to-Ser substitution of the Cdk1 phosphorylation sites in Sic1ΔC; (c) The kinase assays demonstrating the Thr-to-Ser substitution effect on the accumulation rate of doubly phosphorylated forms in T33-T48-Sic1ΔC construct; (d) Full-length Sic1 versions were overexpressed under the galactose promoter to assay the ability of cells to degrade Sic1; (e) Representative ITC experiment to analyze the binding of pT33-Sic1ΔC and pS33-Sic1ΔC to Cks1. No binding heat was detected in the case of pS33-Sic1ΔC; (f) The in vivo demonstration of the requirement of Thr as a priming site of multiphosphorylation using western blotting of Phos-Tag SDS-PAA gels. The dynamics of phosphorylation shifts of the non-destructible Sic1ΔC-derived substrate constructs was followed after the release of cells from α-factor arrest. *Partial phosphorylation of sites by an unknown kinase activity in G1; (g) Mutation of threonines in the CDK consensus sites to serines suppressed the Cks1-dependent multisite phosphorylation of Srl3; (h) Mutation of serines of the CDK consensus sites to threonines induced Cks1-dependent potentiation of multisite phosphorylation of Swi5. The kinase assays in ‘g’ and ‘h’ were performed according to the same protocol as in case of the ones presented in Fig. 1a. The uncropped scans are provided in Supplementary Figure 8.