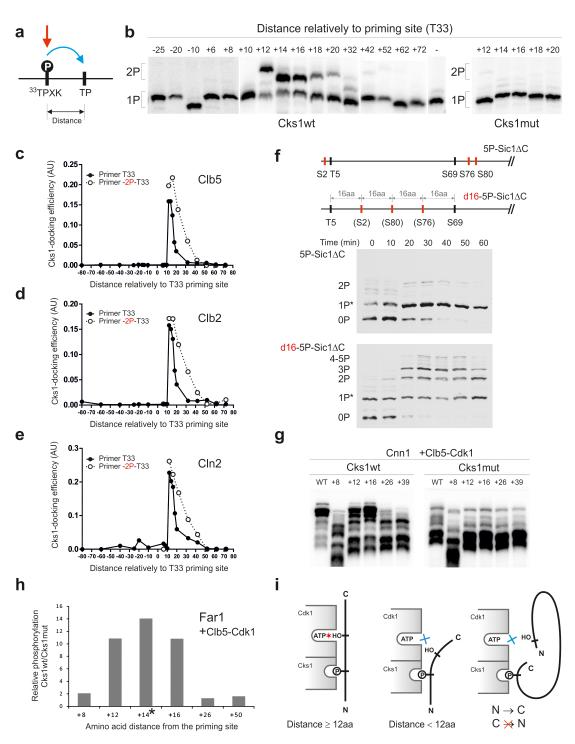
Figure 3. Analysis of the influence of distance between the priming phosphorylation site and the secondary phosphorylation site.
(a) A scheme explaining the positional variation of the secondary site along the Sic1 polypeptide; (b) The autoradiographs of Phos-Tag gels showing the phosphorylation of constructs with varied distances between the priming site (T33) and the secondary site using Clb5-Cdk1. The last lane of the left panel shows the construct containing only the priming site T33; (c-e) The quantified profiles of the relative accumulation rates of doubly phosphorylated forms at different distances between the priming sites and the secondary sites using Clb5-Cdk1 (c), Clb2- Cdk1 (d), and Cln2-Cdk1 (e); (f) The demonstration of the requirement of optimal distances between the phosphorylation sites in vivo by following the phosphorylation shifts in Sic1ΔC-derived constructs by western blotting after the release of cells from α-factor arrest; (g) Distance-dependence in phosphorylation of Cnn1. A secondary phosphorylation site based on site T42 was introduced at different distances from the potential priming site T3; (h) Distance-dependence in phosphorylation of Far1 analyzed by mass spectrometry. A segment containing a secondary phosphorylation site was introduced at different positions relatively to a potential priming site T3 (see Supplementary Fig. 4e-g, Supplementary Table 2). The asterisk indicates that the +14 phosphopeptide of the Cks1mut incubation was not detected, and therefore, the Cks1wt/Cks1mut ratio is large but not precisely measurable; (i) A scheme explaining the optimal distance requirements and the N-to-C-directionality in the Cks1-dependent phosphorylation step. The uncropped scans are provided in Supplementary Figure 8.