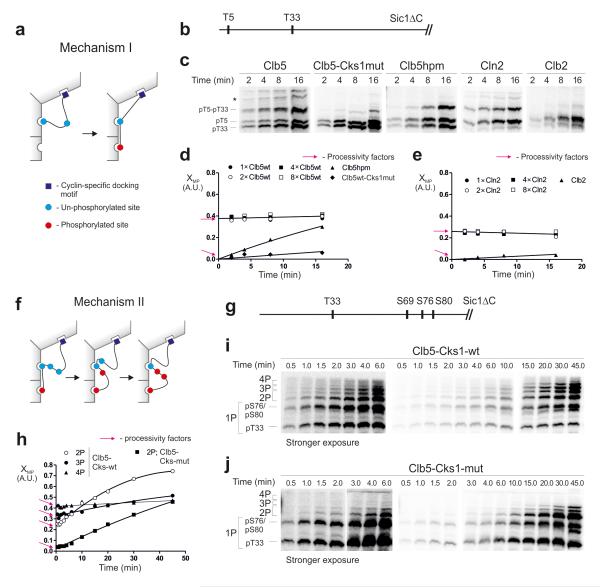
Figure 6. Analysis of the processivity of multi-phosphorylation.
(a) A mechanism in which the phosphorylation of a priming site is followed by the phosphorylation of the secondary site without dissociation of the substrate from the enzyme; (b) Scheme of the substrate construct T5-T33-Sic1ΔC; (c) The time courses showing early stages of the accumulation of doubly phosphorylated forms, separated by Phos-Tag. * unidentified phospho-forms; (d, e) Graphs showing the relative accumulation patterns of doubly phosphorylated forms in the experiments exemplified in panel ‘c’. The y-axis (multi-phosphorylation factor, XMP) shows the ratio of 2P/(1P+2P), where 2P and 1P are the quantified amounts of the doubly and singly (pT5-T33-Sic1ΔC) phosphorylated species, respectively. The y-intercepts are the estimates of the processivity factors, representing the immediate (without a lag period) appearance of doubly phosphorylated forms (see Supplementary Fig. 6). For Clb5 and Cln2 the XMP value does not depend on enzyme concentration, which is another hallmark of processivity (Supplementary Fig. 6). A similar experiment was performed for a two-site substrate construct, T33+12TP-Sic1ΔC (Supplementary Fig.7b). (f) A scheme for the second major processive mechanism. The phosphorylated priming site when docked to the Cks1 pocket can processively phosphorylate several secondary sites without dissociation; (g-j) Analysis of Mechanism II using a construct T33-S69-S76-S80-Sic1ΔC, in which T33 is a priming site. The full time course was used in this case to facilitate broader analysis of the phosphorylated forms. The intercepts provide the estimates for the processivity factors at each step. The uncropped scans are provided in Supplementary Figure 8.