FIGURE 3. Conformation of the nucleotide binding pocket of acto-MV FlAsH as a function of Mg and temperature in the presence of mantdADP.
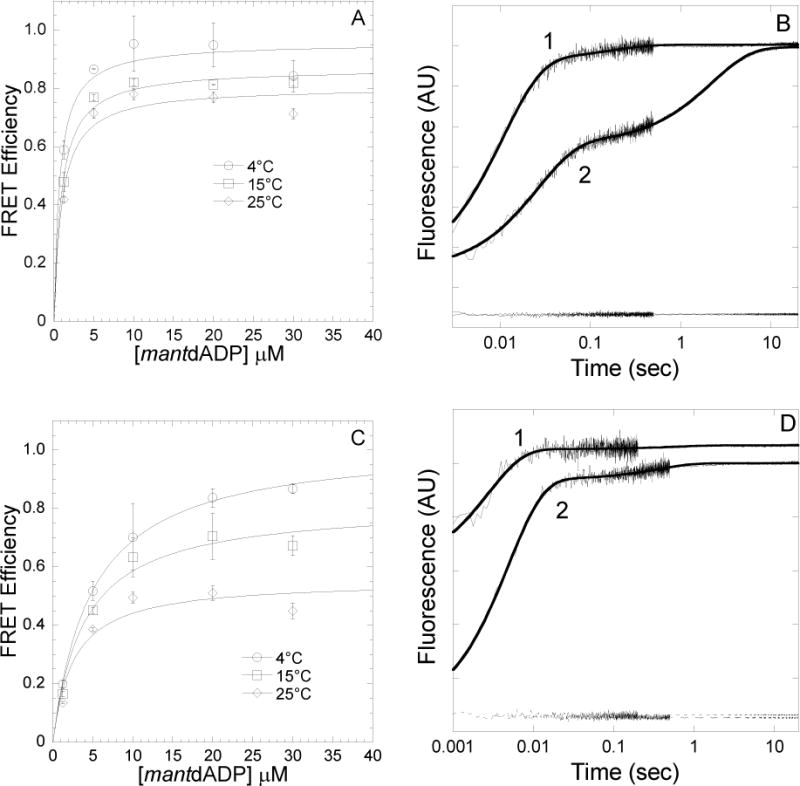
FRET efficiency was determined in a stopped-flow by mixing acto-MV FlAsH with increasing concentrations of mantdADP and measuring the acceptor enhancement. The FRET efficiency was measured at 4, 15 and 25°C in the presence of 2mM MgCl2(A) and 4mM EDTA (C). The data were fit to a hyperbolic binding function to determine the maximum FRET efficiency. Error bars indicate SD from at least two separate experiments done with at least two different protein preparations. Representative fluorescence traces of mantdADP (30μM) binding to actomyosin V FlAsH (0.25μM) in the presence of 4mM EDTA (1) or 2mM MgCl2(2) at 4°C (B) or 25°C (D) are shown. The traces are fit to a double exponential function in all cases. The acceptor alone (actomyosin V FlAsH) traces are also shown at the bottom of the graph.
