FIGURE 4. Kinetics of mantdADP dissociation from actomyosin V FlAsH in the presence and absence of Mg.
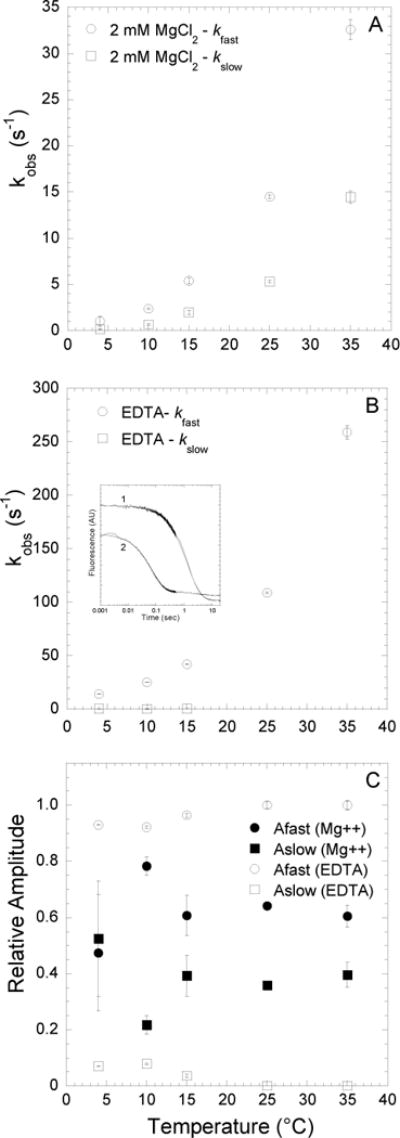
Fluorescence transients were monitored by following the acceptor enhancement after actomyosin V FlAsH complexed with mantdADP was rapidly mixed with saturating ATP. A, At 2mM MgCl2, the dissociation of mantdADP from actomyosin V FlAsH was fit to a double exponential function with the fast and slow phases plotted as a function of temperature. B, In the presence of 4mM EDTA, the fluorescence transients of mantdADP dissociation were fit to a double exponential function at lower temperatures (4–15°C) and single exponential function at higher temperatures (25–35°C). The fast and slow phases are plotted as a function of temperature. Inset shows representative fluorescence transients of mantdADP dissociation in the presence of 2 mM MgCl2 (trace 1) and 4 mM EDTA (trace 2) at 4°C plotted on a log scale and fit to a double exponential function. C, Relative amplitudes of the fast and slow phases, in the presence of 2mM MgCl2 or 4mM EDTA are plotted as a function of temperature.
