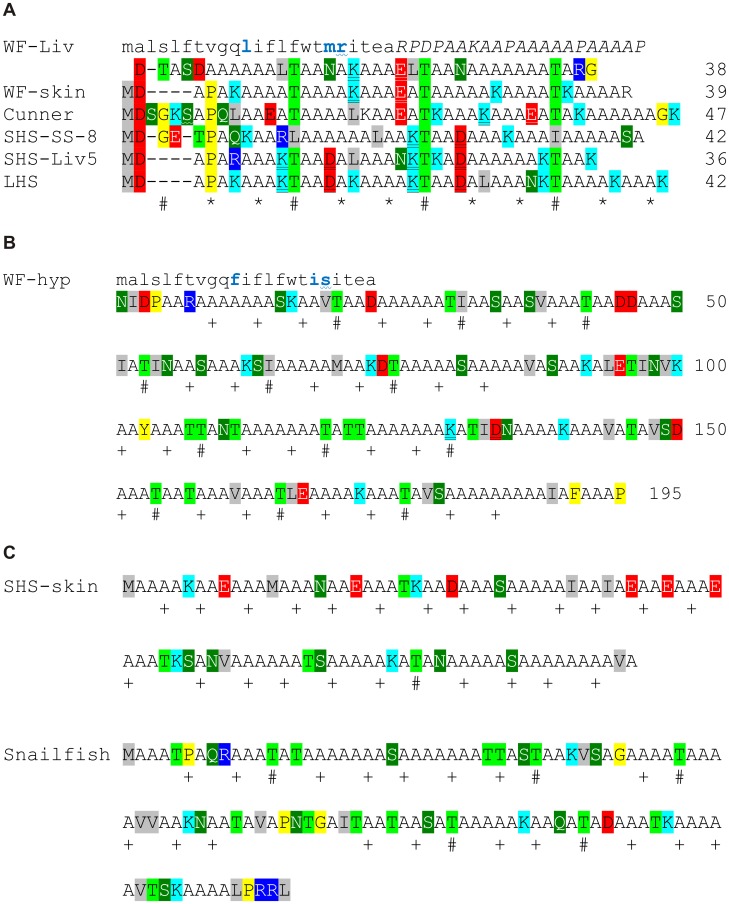
Figure 3. Representative type I AFPs showing their diversity both within species and between species.
Symbols and coloring are as in Fig. 2A. A) Alignment of smaller skin and circulatory isoforms from winter flounder liver (WF-Liv, M63478.1) and skin (WF-skin, M63478.1), cunner (JF937681.2), shorthorn sculpin (SHS) SS-8 [38]and Liv5, longhorn sculpin (LHS, AF306348.1) and cunner (JF937681.2). Only WF-Liv possesses a signal peptide (lower case font, difference relative to WF-hyp in blue) and pro-peptide (italics) which is shown on the line above the mature AFP sequence. Amino acids encoded by codons interrupted by an intron in the cunner [71] and flounder liver sequences are indicated with a wavy underline. The intron within the flounder skin gene lies within the 5′ UTR. B) Sequence of the hyperactive type I AFP from winter flounder (WF-hyp, EU188795.1) denoted as in Fig. 2A. This circulating isoform is dimeric and possesses a signal peptide (lower case font) but no pro-sequence. C) Sequence of the two atypical type I AFPs of intermediate length from shorthorn sculpin skin (SHS-skin, AF305502.1) and dusky snailfish (AY455863.1). Thr is seldom found in position i of the 11 aa i, i+4, i+8 pattern of ice-binding residues and this pattern is not necessarily continuous in these longer AFPs. Neither AFP possesses a signal peptide or prosequence.