Abstract
Purpose
This study investigated the impact of prognostic variables, including the distance a patient lives from a transplant center, on the outcome of autologous hematopoietic stem cell transplantation (ASCT) for multiple myeloma.
Methods
This retrospective analysis included 77 myeloma patients who received an ASCT at Dartmouth Hitchcock Medical Center between 1996 and 2009, 70 of whom were treated between 2002 and 2009. Using linear regression and univariate analysis, we examined the impact of distance from the transplant center on survival. Kaplan-Meier curves identified overall and event-free survival. An association between distance from the transplant center and survival was examined using Cox regression analysis, while adjusting for patient-, disease-, and treatment-related variables.
Results
Increasing distance from the transplant center correlated with improved overall survival (P=.004), but had no impact on disease-free survival (P=.26).
Conclusions
These results suggest that the distance from a transplant center should not be a barrier to ASCT for eligible patients with multiple myeloma.
Keywords: Thrombotic, thrombocytopenia, purpura, ADAMTS13, microangiopathic, hemolytic
Introduction
Multiple myeloma (MM) is an incurable malignancy of plasma cells. It is the second most common blood cancer, comprising 1% of all cancers. In 2009, there were 20,580 new cases of MM, resulting in 10,580 deaths.1 If untreated, the median survival for symptomatic patients is approximately 1 year.2 High-dose chemotherapy followed by autologous stem cell transplant (ASCT) increases the median overall survival (OS) to more than 5 years.3
Although ASCT can offer improved survival for patients with myeloma, the therapy is associated with risks, including a 3–5% mortality rate.3 Hence, research has been directed toward identifying the ideal patient population and setting for this treatment. For a successful clinical transplant outcome, specialized care in a tertiary care setting is required.4,5 The majority of transplant centers in the United States are located in high-volume, urban areas, and patients in rural communities often travel long distances for access to this specialized care.6 One prior study showed increased mortality following transplant for patients living in rural areas.7 However, changes in supportive care and improved initial therapy for MM may impact the referral populations and transplant outcomes for patients in rural settings.
We conducted a retrospective analysis to investigate possible disparities in survival, based on the distance a patient lives from a transplant center. We examined the impact of the distance lived from a transplant center on OS and disease-free survival (DFS) rates. We hypothesized that the distance a patient lives from a transplant center would be associated with increased mortality following transplant, when adjusting for other factors like age, type of myeloma, and use of novel agents.
Methods
Patient Cohort
Patients were identified from the Dartmouth Hitchcock Medical Center (DHMC) transplant registry. DHMC is a large, tertiary care facility with a National Cancer Institute–designated comprehensive cancer center located in rural New Hampshire. This database contains patients who received an ASCT at DHMC from 1996 to January 2009, with 70 of those transplants conducted between 2002 and 2009. A total of 77 consecutive myeloma patients who received a conditioning regimen of melphalan followed by an ASCT were identified.
Data Acquisition
A retrospective chart review was performed by physicians to collect information on patient demographics and each patient's clinical course. Zip codes identified the distance from the patients home to the transplant center, using the web site www.melissadata.com. Zip codes were collected from patient-reported addresses at the time of transplant. Additional patient prognostic factors were identified, including age, immunoglobulin type, time interval from diagnosis to transplant, exposure to novel agents prior to transplant, and disease stage at diagnosis. Novel agents were defined as thalidomide (Thalomid, Celgene), lenalidomide (Revlimid, Celgene), and bortezomib (Velcade, Millennium Pharmaceuticals). A database was constructed using prognostic factors and the distance patients lived from the transplant center.
Defining Primary Outcomes
The primary objective was to determine the OS of the cohort evaluated at 2 endpoints, namely the interval from transplant to death (from any cause) and the interval from the time of initial diagnosis to death (from any cause). The second outcome was defined as progression-free survival (PFS), which was also evaluated using 2 time frames: the time from transplant to relapse/progression and the time from diagnosis to relapse/progression.
Statistical Analysis
A univariate Cox model was used to study the relationship between survival, both OS and PFS, and prognostic variables impacting survival. The age of each patient and the distance a patient lived from the transplant center were examined as continuous variables. The median values of the continuous variables were computed. Once the distance from the transplant center for each patient was identified, the median was used to divide the cohort into 2 groups: patients living more than 50 miles from the transplant center and patients living closer than 50 miles. The type of myeloma was divided into 2 groups: immunoglobulin G (IgG) versus all other subtypes. The status at transplant was divided into groups: partial response (PR) versus other disease states, including progressive disease and stable disease, or complete remission (CR), as defined by the international uniform response criteria.6 Disease stage was characterized into 2 classifications: stage I versus all other stages, using the International Staging System.7
Both OS and PFS were calculated using Kaplan-Meier plots. Multivariate analysis was performed to examine OS and distance from the transplant center, when adjusting for covariates using a Cox regression model. All P values from the survival analysis are calculated based on the Wald test. A result was considered statistically significant if a P value was less than .05.
Results
Patient Demographics
Patient demographics are presented in Table 1. The median age of the patients was 57 years. There were 7 more males than females. Most patients received novel agents prior to transplant and had stage II disease at diagnosis. Only 7 patients entered the transplant in a confirmed CR, and most patients (n=60) were in a PR. IgG myeloma was the most common subtype (n=4l).
Table 1. Patient Demographics, N=77.
| Variable | Median | Range |
|---|---|---|
|
| ||
| Age (years) | 57 | 37–73 |
|
| ||
| Days between dx and transplant | 257 | 135–2,971 |
|
| ||
| Days hospitalized | 17 | 3–26 |
|
| ||
| Distance from center (miles) | 50 | 6–2,225 |
|
| ||
| Performance status (Kamofsky) | 80 | 70–90 |
|
| ||
| Number of lines of therapy | 1 | 1–4 |
|
| ||
| Variable | Number of patients | Percentage |
|
| ||
| Gender | ||
| Male | 42 | 54.5 |
| Female | 35 | 45.5 |
|
| ||
| Exposure to novel agents | ||
| Yes | 49 | 63.6 |
| No | 28 | 36.4 |
|
| ||
| Stage | ||
| I | 27 | 35.1 |
| II | 34 | 44.2 |
| III | 9 | 11.7 |
| Unclassified | 7 | 9.1 |
|
| ||
| Status* | ||
| PR | 60 | 77.9 |
| CR | 7 | 9.1 |
| SD | 5 | 6.5 |
| PD | 3 | 3.9 |
|
| ||
| Type | ||
| IgG | 41 | 53.2 |
| IgD | 2 | 2.6 |
| IgA | 15 | 19.5 |
| IgM | 1 | 1.3 |
| Non-secretory | 16 | 20.8 |
| Unclassified | 1 | 1.3 |
|
| ||
| Cytogenetics | ||
| Normal | 38 | 49.4 |
| Deletion 13 | 7 | 9.1 |
| Complex | 5 | 6.5 |
| Loss of y | 1 | 1.3 |
| Not available | 26 | 33.8 |
Status of disease refers to status at transplant.
CR=complete response; dx=diagnosis; Ig=immunoglobulin; PD=progressive disease; PR=partial response; SD=stable disease.
In Table 2, patient demographics are presented based on distance from the transplant center. The median distance from the transplant center was 50 miles. Thirty-eight patients lived farther than 50 miles away. Of these 38 patients, the median age was 55 years. Novel agents were used in 69% (n=26) of these patients. The median time between diagnosis and transplant was 266 days. Of the 39 patients living closer than 50 miles, the median age was 59 years. Novel agents were used in 60% (n=23) of these patients, and the median time between diagnosis and transplant was 238 days. There were no statistically significant differences between the groups.
Table 2. Patient Demographics Via Distance.
| Variable | <50 miles away (n=39) | >50 miles away (n=38) | P value |
|---|---|---|---|
| Median age (range) | 59 (45–71) | 55 (43–73) | .42 |
| Median days hospitalized (range) | 17 (4–26) | 17 (3–21) | .14 |
| Novel agents, n (%) | 23 (60%) | 26 (69%) | .58 |
| Lines of prior therapy (range) | 1(1–4) | 1 (1–2) | .40 |
| Karnofsky performance score (range) | 80 (70–90) | 85 (70–90) | .47 |
| Days between diagnosis and transplant, median (range) | 238 (145–2971) | 266(143–2,519) | .88 |
Overall and Progression-Free Survival
Figure 1 identifies OS and PFS from the date of transplant. For the total patient cohort, the median OS was 75 months (range, 0.5–142 months) and the median PFS was 22 months (range, 2.5–142 months). There was 1 (1.3%) transplant-associated death during the study period. When evaluating survival from the date of diagnosis, OS remained improved in patients living farther from the transplant center (P=.001).
Figure 1.
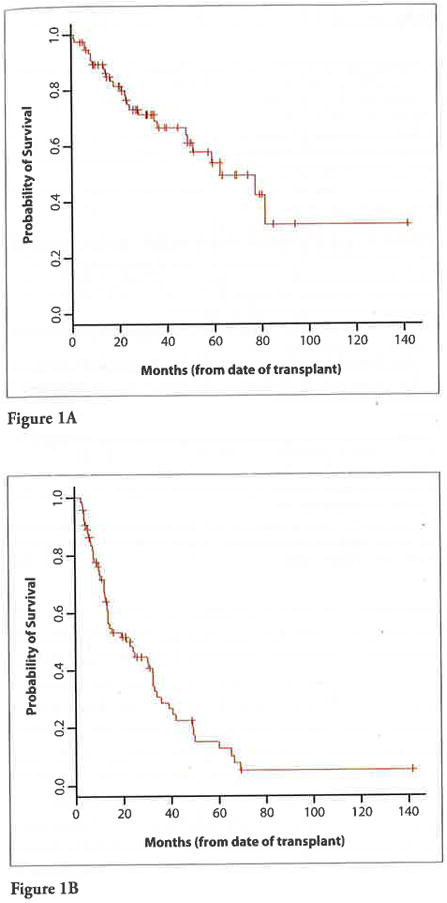
A. Overall survival from the date of transplant.
B. Progression-free survival from the date of transplant.
The results of the multivariate analysis showed that patients living farther from the transplant center had improved OS from the time of diagnosis when viewed as a continuous variable, even when controlled for the combined potential confounders of age, time between diagnosis and transplant, disease type, status, stage, and exposure to novel agents (P=.012). Although patients traveling longer distances tended to be younger and had increased exposure to novel agents, this did not impact survival when analyzed using multivariate analysis.
Distance From the Transplant Center and Correlation With Survival
The results of the univariate analysis revealed that patients traveling greater distances experienced improved overall survival from the time of transplant, when viewed as a continuous variable (P=.004). PFS did not correlate with distance from the transplant center (P=.26). When patients were divided into those living farther than the mean distance of 50 miles and those living less than the distance of 50 miles from the transplant center, an improved survival was seen in patients who lived more than 50 miles away (Figure 2). For patients living more than 50 miles from the hospital, the median OS was 81.6 months compared to 50.4 months for patients living less than 50 miles away (P=.03).
Figure 2.
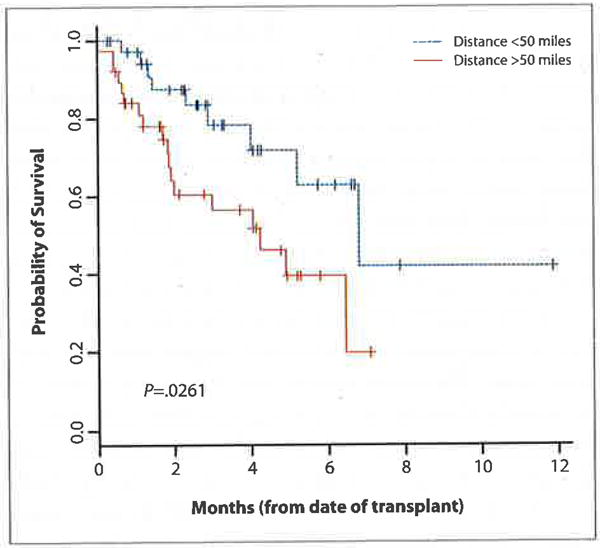
Overall survival from time of transplant versus distance.
Evaluation of Other Prognostic Variables
Patients with IgG myeloma showed a trend toward improved OS when compared to IgA, IgM, IgD, and non-secretory myeloma combined (P=.05). Neither stage (P=.80), age (P=.32), nor time between diagnosis and transplant (P=.25) correlated with survival from the time of transplant, though PFS from the time of diagnosis increased with longer duration between diagnosis and transplant (P=.003). Older patients showed a trend toward decreased PFS (P=.06). Median PFS for patients receiving novel agents was 18 months, versus 30 months for those not receiving novel agents (P=.06). There was no difference in OS (P=.9) or in time from diagnosis to transplant between those groups receiving novel agents and those not receiving novel agents (P=.33).
Discussion
Given concerns about access to care in rural communities and transplant outcomes,8,9 we conducted a retrospective chart review to investigate the impact of the distance a patient lives from a transplant center on OS and DFS in patients undergoing ASCT for MM. The results of out exploratory study show improved OS and DFS for patients living farther from the transplant center, suggesting that distance alone need not be a barrier to transplant.
The improvement in survival may be due to a referral bias in patients being sent for an ASCT. Since patients in this study were the same age regardless of distance traveled, this potential referral bias cannot be attributed to age alone, but may reflect a healthier and more motivated group of patients who are capable of sustaining the long, frequent commutes required for referral to a transplant center.10 For patients living closet to the transplant center, the reduced travel burden may allow more patients—such as those with inferior performance status—to pursue transplant.
Potentially, increased access to medical care for patients living closer to the hospital presents a unique set of risks and complications. For example, patients living closet to a hospital have higher rates of chronic medical illnesses and are more likely to be hospitalized for reasons other than their illness.11 For the transplant population, this potentially translates to a higher likelihood of transplant for patients with chronic medical illnesses and myeloma who live closer to a bone marrow transplant center versus those living farther away. An increased rate of chronic medical conditions places these patients in a higher-risk category for transplant and for complications from hospitalization, such as hospital-acquired infections and resistant infections.
Our study is limited by a relatively small number of transplants that occurred largely over a 7-year time span. The small sample size limits our ability to accurately identify confounding variables and perform multivariate analysis. Additionally, it is possible that changes in therapy or supportive care during the study period may have confounded results. While our results suggest that distance alone need not be a barrier to transplant, this is an exploratory study needing further validation to fully understand the impact of the distance a patient lives from a transplant center on the outcomes in ASCT for MM.
Acknowledgments
This study was approved by the Internal Review Board at Dartmouth Hitchcock Medical Center.
Contributor Information
Dr. Brea C. Lipe, The Division of Hematology and Oncology at the University of Kansas Medical Center in Westwood, Kansas.
Dr. Frederick Lansigan, The Section of Hematology and Oncology at Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
Dr. Jiang Gui, The Norris Cotton Cancer Center at Dartmouth Hitchcock Medical Center.
Dr. Kenneth Meehan, The Section of Hematology and Oncology at Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire.
References
- 1.American Cancer Society. Cancer Facts and Figures 2009. Atlanta, Ga: American Cancer Society; 2009. [Accessed June 16, 2009]. [Google Scholar]
- 2.Osgood EE. The survival time of patients with plasmacytic myeloma. Cancer Chemother Rep. 1960;9:1–10. [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. New Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Loberiza FR, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- 5.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 6.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1–7. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 7.Greipp JR, San Miguel J, Crowley JJ, et al. International system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 8.Edelman MA, Menz BL. Selected comparison and implications of a national rural and urban survey on health care access, demographics, and policy issues. J Rural Health. 1996;12:197–205. doi: 10.1111/j.1748-0361.1996.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 9.Rao K, Darrington DI, Schumacher JJ, et al. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Bial Blood marrow Transplant. 2007;13:1508–1514. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg ER, Chute CG, Stukel T, et al. Social and economic factors in the choice of lung cancer treatment: a population based study in 2 rural states. New Engl J Med. 1988;388:612–617. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 11.Goodman DC, Fisher E, Stukel KA, et al. The distance to community medical care and the likelihood of hospitalization: is closer always better? Am J of Public Health. 1997;87:1144–1150. doi: 10.2105/ajph.87.7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]


