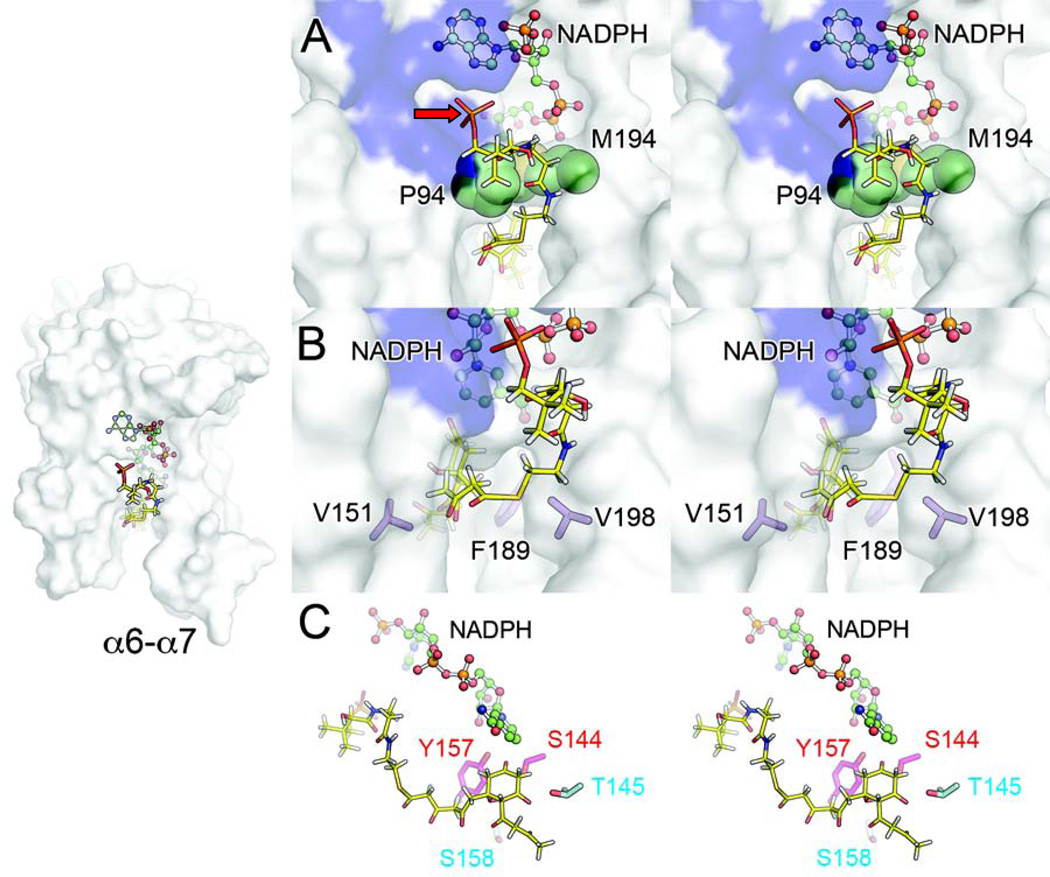
Figure 5. Stereo Images of Proposed Interactions between Polyketide and Wild-Type ActKR.
The polyketide chain is represented in yellow and actKR in transparent white surface.
(A) Ligand docking results suggest that after the PPT phosphate binds within the arginine patch groove (blue surface), the polyketide chain enters the substrate pocket underneath the gate residues P94 and M194 (green spheres) in order to access the active site.
(B) The polyketide intermediate is steered toward the active site by the bulky residues V151, F189, and V198 (purple sticks), which presumably constrain chain movement.
(C) Ligand docking also suggests that T145 (teal) may stabilize the C11-oxyanion after C12 deprotonation through hydrogen-bonding, thus lowering the C12-proton pKa and allowing C7-C12 cyclization to occur. S158 (teal) may stabilize the resulting C7-oxyanion through water-mediated hydrogen-bonding. Both T145 and S158 are highly conserved among type II KRs.