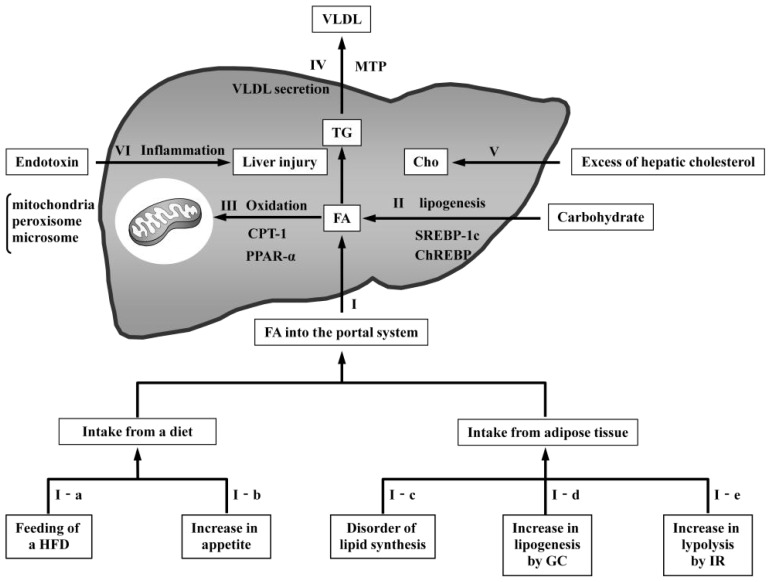
Figure 2.
Mechanisms of NAFLD pathogenesis in rodent models. Dietary triglycerides (TG) are transported in the circulation as chylomicrons. The TG in chylomicrons are degenerated into fatty acids (FA) by lipoprotein lipase (LPL) and delivered to the liver. Furthermore, FA degenerated in adipose tissue are also delivered to the liver. (I) Increased FA from dietary intake or adipose tissue cause fatty changes in the liver. (I-a) Feeding of a high-fat diet (HFD) induces an increased supply of FA; (I-b) increased appetite causes an increased supply of FA as a result of excess dietary intake; (I-c) disordered lipid synthesis in adipose tissue places additional FA directly into the portal system; (I-d) high circulating glucocorticoid (GC) levels cause increased FA induced by lipogenesis; and (I-e) resistance to the antilipolytic action of insulin in adipose tissue results in the excessive release of FA; (II) Hepatic FA are synthesized from carbohydrates through pathways regulated by sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate response element-binding protein (ChREBP). The overexpression of these proteins produces excessive FA in the liver, leading to fatty changes; (III) FA oxidation occurs in the mitochondria, peroxisomes, and microsomes. Carnitine palmitoyltransferase 1 (CPT1) controls the transport of acyl-CoA into the mitochondria. Peroxisome proliferator-activated receptor alpha (PPARα) is a transcription factor that regulates oxidation pathways. Reduced oxidation results in fatty changes by increasing hepatic FA; (IV) Hepatic TG are released from the liver as components of VLDL. Microsomal triglyceride transfer protein (MTP) regulates the synthesis of VLDL. Decreased VLDL secretion causes the accumulation of FA in the liver; (V) Hepatic cholesterol accumulation leads to calcium depletion and endoplasmic reticulum (ER) stress, with the activation of the unfolded protein response and ER stress-induced apoptosis; (VI) Low-dose endotoxin induces liver injury in HFD-induced steatosis.