Abstract
Interleukin (IL)-3, a multilineage hematopoietic growth factor, is implicated in the regulation of osteoclastogenesis. However, the role of IL-3 in osteoclastogenesis remains controversial; whereas early studies showed that IL-3 stimulates osteoclastogenesis, recent investigations demonstrated that IL-3 inhibits osteoclast formation. The objective of this work is to further address the role of IL-3 in osteoclastogenesis. We found that IL-3 treatment of bone marrow cells generated a population of cells capable of differentiating into osteoclasts in tissue culture dishes in response to the stimulation of the monocyte/macrophage-colony stimulating factor (M-CSF) and the receptor activator of nuclear factor kappa B ligand (RANKL). The IL-3-dependent hematopoietic cells were able to further proliferate and differentiate in response to M-CSF stimulation and the resulting cells were also capable of forming osteoclasts with M-CSF and RANKL treatment. Interestingly, IL-3 inhibits M-CSF-/RANKL-induced differentiation of the IL-3-dependent hematopoietic cells into osteoclasts. The flow cytometry analysis indicates that while IL-3 treatment of bone marrow cells slightly affected the percentage of osteoclast precursors in the surviving populations, it considerably increased the percentage of osteoclast precursors in the populations after subsequent M-CSF treatment. Moreover, osteoclasts derived from IL-3-dependent hematopoietic cells were fully functional. Thus, we conclude that IL-3 plays dual roles in osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process. These findings provide a better understanding of the role of IL-3 in osteoclastogenesis.
Keywords: Interleukin-3, Osteoclastogenesis, Osteoclast progenitor, Osteoclast precursor, M-CSF, RANKL
1. Introduction
Osteoclasts, the bone-resorbing cells, play an important role in skeletal development and adult bone remodeling [1,2]. Osteoclasts differentiate from hematopoietic cells of the monocyte/macrophage lineage involving several different stages [3]. Hematopoietic stem cells (HSC) give rise to common myeloid progenitors (CMP) with stimulation of various factors including stem cell factor (SCF), IL-3 and interleukin 6 (IL-6). IL-3 and/or the granulocyte/macrophage-colony stimulating factor (GM-CSF) further promote development of CMP into granulocyte/macrophage progenitors (GMP). CMP and GMP are collectively considered osteoclast progenitors. M-CSF then promotes GMP to differentiate into cells of the monocyte/macrophage lineage [4,5], which are osteoclast precursors. M-CSF and RANKL are two essential and sufficient factors driving osteoclast precursors to differentiate into osteoclasts [6,7].
IL-3 is a multilineage hematopoietic growth factor that promotes the proliferation, differentiation and/or survival of early multilineage hematopoietic progenitors [8]. In particular, this cytokine plays a key role in stimulating the proliferation and survival of myeloid precursors. By the early 1980s, it had been well established that osteoclasts differentiate from hematopoietic cells of the monocyte/macrophage lineage [9]. Given the role of IL-3 in the proliferation and survival of myeloid precursors, a number of groups investigated the potential role of IL-3 in osteoclastogenesis in vitro in the late 1980s [10,11,12,13,14]. Collectively, these early investigations demonstrated that IL-3 stimulates osteoclastogenesis in vitro using either organ cultures or whole bone marrow cultures.
Intriguingly, numerous recent studies showed that IL-3 inhibits osteoclast formation in in vitro osteoclastogenesis assays in which osteoclast precursors were treated with the two essential osteoclast factors M-CSF and RANKL [15,16,17,18,19]. Importantly, these studies indicate that the inhibitory regulation in the osteoclastogenesis assays results from the direct effect of IL-3 on osteoclast precursors. Thus, the role of IL-3 in osteoclastogenesis remains controversial.
In this study, we seek to further address the role of IL-3 in osteoclastogenesis. Our results demonstrate that IL-3 stimulates the development of osteoclast progenitors from bone marrow cells, but it inhibits differentiation of osteoclast precursors into osteoclasts.
2. Materials and methods
2.1. Chemicals and biological reagents
Recombinant mouse IL-3 was obtained from R&D System, Inc. (Minneapolis, MN). Mouse M-CSF was prepared as culture supernatants from CMG14-12 cells, an M-CSF-producing cell line kindly provided by Dr. Sunao Takeshita [20]. Recombinant GST-RANKL was prepared in our laboratory as previously described [21]. Phycoerythrin (PE)-conjugated anti-mouse CD11b antibody and allophycocyanin (APC)-conjugated rat IgG2a k isotype control antibody were obtained from eBioscience (San Diego, CA). APC-conjugated anti-mouse CD115 (c-Fms) antibody was purchased from BioLegend (San Diego, CA). PE-conjugated rat IgG2a k isotype control antibody was from BD Pharmingen (San Jose, CA).
2.2. Preparation and culture of mouse bone marrow cells
C57BL/6 mice were obtained from Harlan Industries (Indianapolis, IN). The experiments involving mice were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Bone marrow cells were obtained from long bones of young (4–6 week-old) mice and cultured in α-minimal essential medium (α-MEM) containing 10% heat-inactivated fetal bovine serum (FBS) in the presence of different factors as indicated in individual experiments.
2.3. In vitro osteoclastogenesis assay
Different numbers of cells as specified in individual assays were seeded in wells of 24-well tissue culture plates and cultured in α-MEM supplemented with 44ng/ml M-CSF plus RANKL 100ng/ml for 5 days. The osteoclastogenesis cultures were then stained for tartrate resistant acid phosphatase (TRAP) activity with the Leukocyte Acid Phosphatase kit (387-A) from Sigma-Aldrich (St. Louis, MO).
2.4. Flow cytometry
1×106 cells were washed with cold phosphate-buffered buffers (PBS) and resuspended in 200μl blocking buffer (PBS/0.5% BSA/0.1% Azide) containing 2.4G2 antibody (5μg/mL) for 30min on ice. Cells were then washed with 500μl PBS/azide before addition of 0.5ul PE-conjugated anti-CD11b antibody and APC-conjugated anti-CD115 antibody or corresponding control IgG antibodies. The mixtures were incubated on ice for 30 min under dim light and then washed with 1 mL cold PBS/azide twice. Cells were fixed with 400μl cold 1% formaldehyde and analyzed using Accuri C6 Flow Cytometor.
2.5. In vitro bone resorption assay
Cells are seeded on bovine bone slices in 24-well culture plates and cultured as indicated in individual experiments to promote osteoclastogenesis and bone resorption. Bone slices were harvested and cells were removed with 0.25M ammonium hydroxide and mechanical agitation. Bone pits were analyzed by scanning electron microscopy (SEM) using a Philips 515 scanning microscope.
2.6. Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the cells using TRIzol reagent from Invitrogen (Carlsbad, CA) and 1μg of total RNA was reversed-transcribed to cDNA with oligo(dT) using the ThermoScriptTM RT-PCR system (Invitrogen). Semi-quantitative RT-PCR experiments were performed using the primers and conditions described previously [22].
2.7. Statistical analysis
Cell numbers are presented as mean ± S.D. Statistical significance was determined using Student t test. A p value less than 0.05 was considered significant.
3. Results
3.1. IL-3 treatment of mouse bone marrow cells generates a population of cells capable of forming osteoclasts
Although recent studies demonstrated that IL-3 exerts inhibitory effects on the osteoclastogenic process [15,16,17,18,19], numerous early investigations involving organ cultures or whole bone marrow cultures showed that IL-3 promotes osteoclastogenesis [10,11,12,13,14]. These conflicting findings suggest that IL-3 may exert dual effects on osteoclastogenesis; while IL-3 inhibits the differentiation of osteoclast precursors into mature osteoclasts, it may also promote the differentiation, proliferation and/or survival of osteoclast progenitors or precursors since it has been well established that IL-3 supports the growth and development of early hematopoietic multilineage progenitors in cell culture [8,23]. Thus, our first experiment aimed to address this possibility. As depicted in Fig. 1A, we cultured whole bone marrow cells in tissue culture dishes overnight to remove bone marrow stromal cells. Then, nonadherent cells were moved to new tissue culture dishes, in which the cells continued to be cultured for 3 or 6 days without IL-3 (control) or with IL-3. While no cells survived in the control culture dishes after the 6-day culturing, the IL-3-treatment gave rise to a significant number of surviving cells (Fig. 1B). To determine whether the surviving cells can form osteoclasts, they were seeded at different densities in 24-well tissue culture dishes and then treated with M-CSF and RANKL for 5 days. As shown in Fig. 1C, M-CSF and RANKL were able to stimulate these cells to form osteoclasts in tissue culture dishes, indicating that IL-3 promotes the differentiation, proliferation and/or survival of osteoclast progenitors or precursors.
Fig. 1.
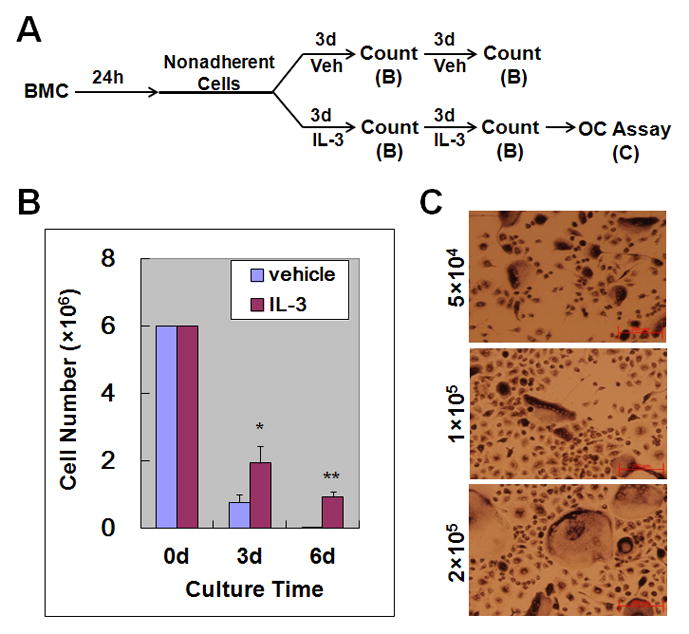
IL-3 treatment of bone marrow cells generates a population of cells capable of forming osteoclasts. (A) A brief description of the experimental procedure. Bone marrow cells (BMC) were cultured for 24 hours (h) in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with vehicle (Veh, i.e., PBS) or IL-3 (1ng/ml) for up to 6 days (d). At d 3 and d 6, surviving cells were counted and quantified in panel B. Cells from the 6d cultures were used to perform osteoclast (OC) assays, and the results are shown in panel C. (B) 6×106 cells were added to one 60-mm tissue culture dish and the assay was performed in triplicate. Data are expressed as mean ± S.D. *, p<0.05; **, p<0.01. (C) Different numbers of surviving cells from the 6d culture described in (A) were seeded in 24-well tissue culture plates and cultured with 44ng/ml M-CSF and 100ng/ml RANKL for 5d. The cultures were then stained for TRAP activity. The assay was independently repeated 3 times and a representative area from each condition is shown.
3.2. M-CSF further enhances the osteoclastogenic potential of IL-3 dependent hematopoietic progenitors and IL-3 inhibits the differentiation of IL-3 dependent hematopoietic progenitors into osteoclasts
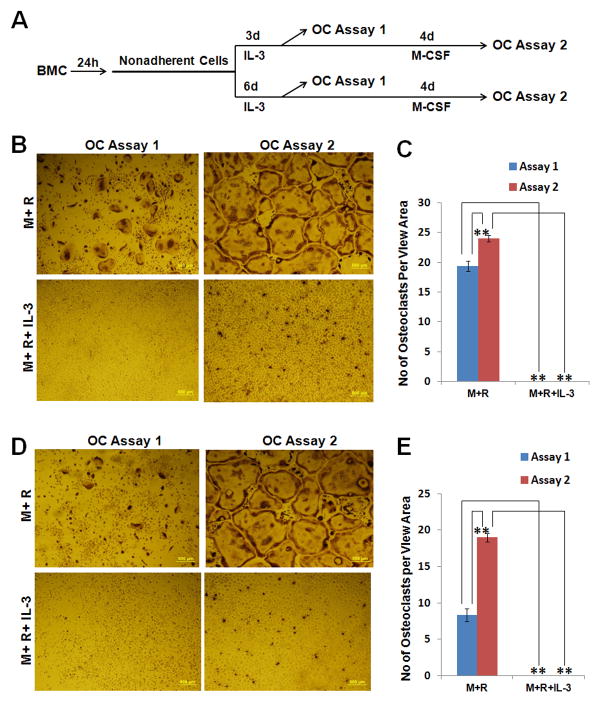
Next, we examined the effect of M-CSF treatment on the osteoclastogenic potential of IL-3 dependent hematopoietic cells. To this end, we repeated the assay in Fig. 1A with IL-3 treatment for 3 or 6 days (Fig. 2A). At day 3, cells were treated with M-CSF and RANKL in the absence or presence of IL-3 to assess their ability to form osteoclasts and the potential effect of IL-3 on the osteoclastogenic process (OC Assay 1, Fig. 2B). The remaining cells were further cultured with M-CSF for 4 days (Fig. 2A) and then used to repeat the same set of assays (OC Assay 2, Fig. 2B). These experiments were also performed with hematopoietic cells resulting from 6-day IL-3 treatment (Fig. 2D). While IL-3 dependent hematopoietic cells were able to form osteoclasts (OC Assay 1, Fig. 2B/2D), subsequent M-CSF treatment of these cells gave rise to much bigger osteoclasts (OC Assay 2, Fig. 2B/2D) as well as more multinucleated TRAP-positive cells (Fig. 2C/2E). Moreover, IL-3 inhibits M-CSF-/RANKL-induced differentiation of IL-3 dependent hematopoietic cells into osteoclasts (Fig. 2B–2E). These findings indicate that a) M-CSF can further enhance the osteoclastogenic potential of IL-3-dependent hematopoietic progenitors and b) IL-3 inhibits the differentiation of IL-3 dependent hematopoietic progenitors into osteoclasts.
Fig. 2.
M-CSF further enhances the osteoclastogenic potential of IL-3-dependent cells and IL-3 inhibits the differentiation of IL-3-dependent progenitors into osteoclasts. (A) A brief description of the experimental procedure. Bone marrow cells (BMC) were cultured for 24 hours (h) in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with IL-3 for 3 or 6 days (d). Then some of the surviving cells from the 3d and 6d cultures were used to perform osteoclast (OC) assay (OC Assay 1), and remaining cells were continued with 220ng/ml M-CSF for 4 days prior to OC assay (OC Assay 2). (B) OC Assay 1 and OC Assay 2 with cells from 3-day IL-3 treatment. The cells were seeded in 24-well tissue culture plates (2.5×104cells/well) and cultured with 44ng/ml M-CSF (M) and 100ng/ml RANKL (R), or with 44ng/ml M and 100ng/ml R plus 1ng/ml IL-3 for 5d. (C) Quantification of assays in B. Multinucleated TRAP-positive cells (>3nuclei) per representative view area (40 × magnification) was counted. Bars show averages of three replicates ±S.D. **, P<0.01. (D) OC Assay 1 and OC Assay 2 with cells from 6-day IL-3 treatment. The assays were performed as in B. (E) Assays in D were quantified as in C. Bars show averages of three replicates ±S.D. **, P<0.01. The cultures were stained for TRAP activity. The assay was independently repeated 2 times and a representative area from each condition is shown.
3.3. IL-3 promotes the development of osteoclast progenitors
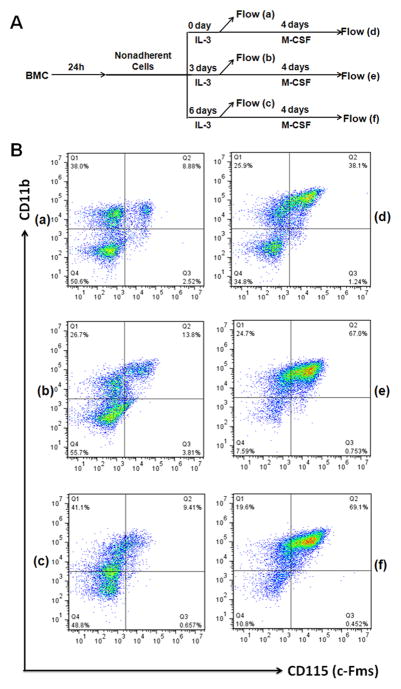
To delineate the mechanism underlying the difference in the osteoclastogenic potential seen in Fig. 2, we next sought to determine the percentage of osteoclast precursors in the IL-3-dependent cell population prior to and after M-CSF stimulation using two known markers for osteoclast precursors, namely, CD11b and CD115 (c-Fms) [24]. To this end, we prepared bone marrow cells without IL-3 treatment or with IL-3 treatment for 3 or 6 days (Fig. 3A). While some of these cells were used to perform flow cytometry assays to assess surface expression of CD11b and CD115, remaining cells were further treated with M-CSF for 4 days prior to flow cytometry assays (Fig. 3A). The data indicate that IL-3 treatment of bone marrow cells slightly affected the percentage of osteoclast precursors with 8.88%, 13.8% and 9.41% osteoclast precursors in bone marrow cells without IL-3 treatment, with 3-day IL-3 treatment, and with or 6-day IL-3 treatment, respectively (a, b and c, Fig. 3). Interestingly, subsequent M-CSF treatment of these bone marrow cells yielded increased percentages of osteoclast precursors with 38.1% osteoclast precursors in bone marrow cells without IL-3 treatment compared to 67.0% and 69.1% osteoclast precursors in bone marrow cells with 3-day IL-3 treatment and with or 6-day IL-3 treatment, respectively (d, e and f, Fig. 3). Thus, IL-3-treated bone marrow cells had more osteoclast progenitors, indicating that IL-3 promotes the development of osteoclast progenitors.
Fig. 3.
IL-3 promotes the development of osteoclast progenitors. (A) A brief description of the experimental procedure. Bone marrow cells (BMC) were cultured for 24 hours (h) in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with IL-3 for 0, 3 or 6 days. Then, some of the surviving cells were used to perform flow cytometric assays to determine surface expression of CD11b and CD115 (c-Fms) (Flow a, b and c), and remaining cells were continued with 220ng/ml M-CSF for 4 days prior to flow cytometric assays (Flow d, e and f). (B) Results of flow cytometric assays (a, b, c, d, e and f) as described in (A). The assay was independently repeated 2 times and similar results were obtained. One set of the data is shown.
3.4. Osteoclasts derived from IL-3-dependent hematopoietic cells are functional
To further establish the role of IL-3 in the development of osteoclast progenitors, we determined whether osteoclasts generated from IL-3-dependent hematopoietic cells have the capacity to resorb bone. Bone marrow cells maintained with IL-3 for 5 or 6 days were seeded on bone slices in a 24-well tissue culture plate and cultured with M-CSF alone (control) or M-CSF and RANKL for 9 days. Then bone slices were processed for SEM analysis (Fig. 4A). The data show that osteoclasts generated from IL-3-dependent hematopoietic progenitors are capable of resorbing bones. In parallel, some bone marrow cells maintained with IL-3 for 5 or 6 days were further cultured with M-CSF for 4 days prior to being used for bone resorption assays (Fig. 4B). We found that osteoclasts derived from IL-3-dependent hematopoietic progenitors with further M-CSF treatment are also functional.
Fig. 4.
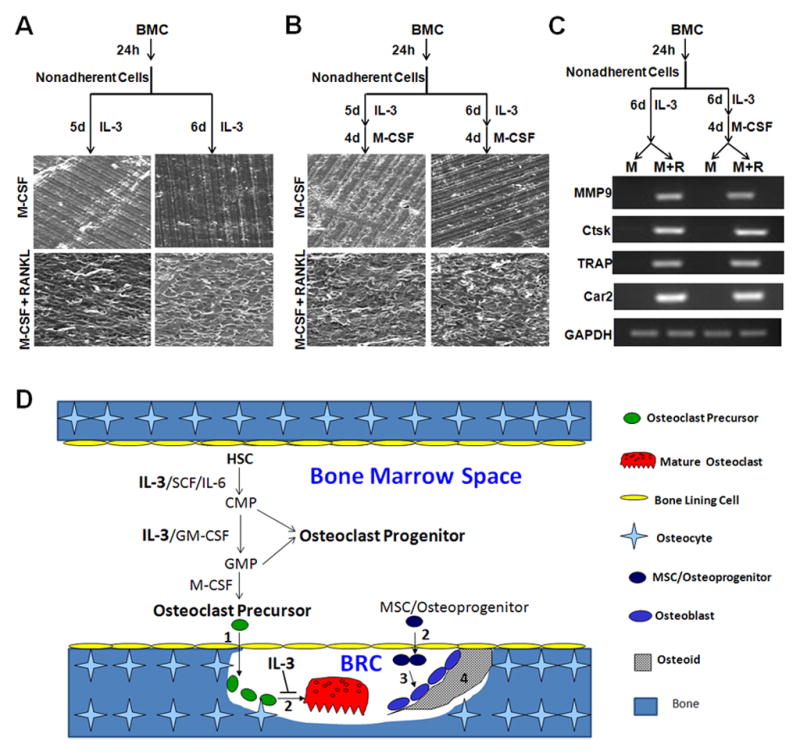
IL-3-dependent cells can form functional osteoclasts and a model for the role of IL-3 in osteoclast biology. (A) Bone marrow cells (BMC) were cultured for 24 hours (h) in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with IL-3 for 5 or 6 days (d). 1×105 of surviving cells were seeded on bone slices in 24-well tissue culture plates and cultured with 44ng/ml M-CSF alone or with 44ng/ml M-CSF and 100ng/ml RANKL for 9d to perform bone resorption assays. (B) BMC were cultured for 24h in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with IL-3 for 5 or 6d, followed by M-CSF treatment (44ng/ml) for 4d. Then 5×104 of cells were seeded on bone slices in 24-well tissue culture plates and cultured with 44ng/ml M-CSF alone or with 44ng/ml M-CSF and 100ng/ml RANKL for 9d to perform bone resorption assays. These assays were independently repeated 2 times and a representative area is shown. (C) BMC were cultured for 24h in tissue culture dishes. Nonadherent cells were then moved to new tissue culture dishes and cultured with IL-3 for 6d. Then, some of the surviving cells were cultured with 44ng/ml M-CSF alone (control) or with 44ng/ml M-CSF and 100ng/ml RANKL for 5d to form osteoclasts. The remaining cells were continued with M-CSF (44ng/ml) for 5d, followed by treatment with M-CSF alone or M-CSF (44ng/ml) and RANKL (100ng/ml) for 5d. The expression of representative osteoclasts genes (MMP9, Ctsk, TRAP and Car2) was assessed by semi-quantitative RT-PCR. GAPDH was used as control. The assay was independently repeated 2 times. (D) A model for the role of IL-3 in osteoclastogenesis in the context of normal bone remodeling.
We next investigated osteoclast gene expression in osteoclasts generated from IL-3-dependent hematopoietic cells. Bone marrow cells cultured with IL-3 for 6 days were seeded in tissue culture dishes with M-CSF alone (control) or M-CSF and RANKL for 5 days. Moreover, bone marrow cells treated with IL-3 for 6 days were switched to M-CSF for 5 days. The resultant cells were then seeded in tissue culture dishes with M-CSF alone (control) or M-CSF and RANKL for 5 days. The expression of four osteoclast genes (Ctsk, cathepsin K; MMP9, matrix metalloproteinase 9; Car2, carbonic anhydrase 2; and TRAP, tartrate acid resistant phosphatase) was assessed by semi-quantitative RT-PCR using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as control. As shown in Fig. 4C, all these osteoclast genes are expressed in IL-3-dependent hematopoietic progenitors treated with M-CSF and RANKL but not in those treated with M-CSF alone. The data demonstrate that osteoclasts generated from IL-3-dependent hematopoietic cells express osteoclast genes, thus further indicating that osteoclasts derived from IL-3-dependent hematopoietic progenitors with further M-CSF treatment are functional.
4. Discussion
In this work, we have carried out various assays to further address the role of IL-3 in osteoclastogenesis. Our key findings are a) IL-3 treatment of bone marrow cells can generate a population of cells capable of forming osteoclasts in tissue culture dishes in response to subsequent M-CSF and RANKL stimulation (Fig. 1); b) M-CSF can further enhance the osteoclastogenic potential of IL-3-dependent hematopoietic progenitors (Fig. 2); c) IL-3 inhibits the differentiation of IL-3-dependent hematopoietic progenitors into osteoclasts (Fig. 2); and d) IL-3 promotes the development of osteoclast progenitors (Fig. 3). Moreover, given the controversy on the role of IL-3 in osteoclastogenesis, we extended our study to confirm that osteoclasts derived from IL-3-dependent hematopoietic cells are functional (Fig. 4A–C).
Our finding is consistent with the conclusions from early studies that IL-3 stimulates osteoclastogenesis in organ or whole bone marrow cultures [10,11,12,13,14], which contained different cell types including bone marrow stromal cells. We used bone marrow cells depleted of bone marrow stromal cells. Thus, our study provides evidence that IL-3 promotes the development of osteoclast progenitors by directly targeting hematopoietic progenitors. Also, we have replicated recent observations that IL-3 inhibits the osteoclastogenic process [15,16,17,18,19]. The opposite effects of IL-3 on different stages of osteoclastogenesis have led to an important question - what is the net effect of IL-3 on osteoclastogenesis and bone remodeling? We feel that this question should be answered in the context of the newly proposed model for bone remodeling. Based on numerous recent studies [25,26,27], it is proposed that osteoclastogenesis occurs within a closed space termed “bone remodeling compartment (BRC)”, which is very vascular and characterized by the presence of a canopy formed by bone lining cells [2] (Fig. 4D). Bone remodeling can be divided into four major stages [2]. Stage 1: initiation of bone remodeling by recruitment of osteoclast precursors into the BRC; Stage 2: bone resorption phase characterized by predominant osteoclast formation and function (bone resorption) with concurrent recruitment of mesenchymal stem cells (MSC)/osteoprogenitors into the BRC; Stage 3: bone formation stage which involve primarily osteoblast differentiation and function (osteoid synthesis); and lastly Stage 4: mineralization of osteoid and termination of bone remodeling.
This newly proposed model highlights the notion that development of osteoclast progenitors/precursors is spatially separated from the formation of mature osteoclasts. Specifically, the development of osteoclast progenitors/precursors occurs within the bone marrow space (Fig. 4D). HSC give rise to CMP with stimulation of various factors such as IL-3, stem cell factor (SCF) and IL-6. IL-3 and/or GM-CSF further promote development of CMP into GMP. CMP and GMP are considered osteoclast progenitors (Fig. 4D). With stimulation by M-CSF, GMP in turn differentiate into cells of the monocyte/macrophage lineage [4,5], which are considered osteoclast precursors. In contrast, osteoclast differentiation occurs within the BRC in which M-CSF and RANKL produced by osteoblasts and/or osteocytes stimulate osteoclast differentiation (Fig. 4D). Given that activated T-cells, but not osteoblasts or osteocytes, are majors sources of IL-3, IL-3 is unlikely to be present in the BRC to have a significant impact on osteoclastogenesis in normal bone remodeling. However, it is possible that IL-3 may play a role in regulating osteoclastogenesis in pathological conditions under which the integrity of the BRC is compromised and/or IL-3-producing cells such as activated T-cells mistakenly enter the BRC.
In conclusion, this work reveals that IL-3 exerts dual effects on osteoclastogenesis in that it promotes the development of osteoclast progenitors but inhibits the osteoclastogenic process. Significantly, these findings not only provide a better understanding of the role of IL-3 in osteoclastogenesis but may also facilitate future studies to delineate the role of IL-3 in the pathogenesis of bone diseases.
Highlights.
IL-3 treatment of bone marrow cells generates a population of hematopoietic cells.
IL-3-dependent hematopoietic cells are capable of differentiating into osteoclasts.
Osteoclasts derived from IL-3-dependent hematopoietic cells are functional.
IL-3 promotes the development of osteoclast progenitors.
IL-3 inhibits the osteoclastogenic process.
Acknowledgments
The work is supported by grant numbers 3087110 and 80171939 (to SW) from the National Natural Science Foundation of China, grant number 11C33150710 (to SW) from the Guangzhou Science and Technology Program, and grant number AR47830 (to XF) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). PQ is supported by a scholarship from the State Scholarship Fund of China Scholarship Council.
Abbreviations
- α-MEM
α-minimal essential medium
- APC
allophycocyanin
- BMC
bone marrow cells
- BRC
bone remodeling compartment
- Car2
carbonic anhydrase 2
- CMP
common myeloid progenitors
- Ctsk
cathepsin K
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GMP
granulocyte/macrophage progenitors
- GM-CSF
granulocyte/macrophage colony stimulating factor
- IL-3
Interleukin 3
- IL-6
interleukin 6
- HSC
hematopoietic stem cells
- M-CSF
monocyte/macrophage-colony stimulating factor
- MSC
mesenchymal stem cells
- MMP9
matrix metalloproteinase 9
- PBS
phosphate-buffered buffers
- PE
phycoerythrin
- RANKL
receptor activator of nuclear factor kappa B ligand
- RT-PCR
reverse transcription-polymerase chain reaction
- SCF
stem cell factor
- SEM
scanning electron microscopy
- TRAP
tartrate resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005;350:1–13. doi: 10.1016/j.gene.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 7.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 8.Yang YC, Clark SC. Interleukin-3: molecular biology and biologic activities. Hematol Oncol Clin North Am. 1989;3:441–452. [PubMed] [Google Scholar]
- 9.Loutit JF, Nisbet NW. The origin of osteoclasts. Immunobiology. 1982;161:193–203. doi: 10.1016/S0171-2985(82)80074-0. [DOI] [PubMed] [Google Scholar]
- 10.Scheven BA, Visser JW, Nijweide PJ. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986;321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo JA, Sousa SL, Fonseca JM, Hock JM, Medlock ES. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Invest. 1987;80:160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton BE, Mayer R. IL-3 induces differentiation of bone marrow precursor cells to osteoclast-like cells. J Immunol. 1989;143:3211–3216. [PubMed] [Google Scholar]
- 13.Hattersley G, Chambers TJ. Effects of interleukin 3 and of granulocyte-macrophage and macrophage colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol. 1990;142:201–209. doi: 10.1002/jcp.1041420125. [DOI] [PubMed] [Google Scholar]
- 14.Shinar DM, Sato M, Rodan GA. The effect of hemopoietic growth factors on the generation of osteoclast-like cells in mouse bone marrow cultures. Endocrinology. 1990;126:1728–1735. doi: 10.1210/endo-126-3-1728. [DOI] [PubMed] [Google Scholar]
- 15.Khapli SM, Mangashetti LS, Yogesha SD, Wani MR. IL-3 acts directly on osteoclast precursors and irreversibly inhibits receptor activator of NF-kappa B ligand-induced osteoclast differentiation by diverting the cells to macrophage lineage. J Immunol. 2003;171:142–151. doi: 10.4049/jimmunol.171.1.142. [DOI] [PubMed] [Google Scholar]
- 16.Yogesha SD, Khapli SM, Wani MR. Interleukin-3 and granulocyte-macrophage colony-stimulating factor inhibits tumor necrosis factor (TNF)-alpha-induced osteoclast differentiation by down-regulation of expression of TNF receptors 1 and 2. J Biol Chem. 2005;280:11759–11769. doi: 10.1074/jbc.M410828200. [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, Barhanpurkar AP, Tomar GB, Srivastava RK, Kour S, Pote ST, Mishra GC, Wani MR. IL-3 inhibits human osteoclastogenesis and bone resorption through downregulation of c-Fms and diverts the cells to dendritic cell lineage. J Immunol. 2010;185:2261–2272. doi: 10.4049/jimmunol.1000015. [DOI] [PubMed] [Google Scholar]
- 18.Khapli SM, Tomar GB, Barhanpurkar AP, Gupta N, Yogesha SD, Pote ST, Wani MR. Irreversible inhibition of RANK expression as a possible mechanism for IL-3 inhibition of RANKL-induced osteoclastogenesis. Biochem Biophys Res Commun. 2010;399:688–693. doi: 10.1016/j.bbrc.2010.07.143. [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Lee MS, Yeon JT, Choi SW, Kim HS, Shim H, Lee SY, Youn BS, Yokota Y, Kim JH, Kwak HB. Inhibitory regulation of osteoclast differentiation by interleukin-3 via regulation of c-Fos and Id protein expression. J Cell Physiol. 2012;227:1851–1860. doi: 10.1002/jcp.22913. [DOI] [PubMed] [Google Scholar]
- 20.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 21.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jules J, Shi Z, Liu J, Xu D, Wang S, Feng X. Receptor activator of NF-{kappa}B (RANK) cytoplasmic IVVY535–538 motif plays an essential role in tumor necrosis factor-{alpha} (TNF)-mediated osteoclastogenesis. J Biol Chem. 2010;285:37427–37435. doi: 10.1074/jbc.M110.149484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YC, Donahue RE, Ogawa M, Clark SC. Molecular cloning and biological characterization of human interleukin-3 (IL-3) Transplant Proc. 1989;21:50–53. [PubMed] [Google Scholar]
- 24.Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res. 2013;28:574–585. doi: 10.1002/jbmr.1760. [DOI] [PubMed] [Google Scholar]
- 26.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 27.Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res. 2001;16:1583–1585. doi: 10.1359/jbmr.2001.16.9.1583. [DOI] [PubMed] [Google Scholar]