Abstract
Objective
To analyze the predictors and patterns of recurrence of melanoma in patients with a negative sentinel lymph node biopsy result.
Design
Retrospective chart review of a prospectively created database of patients with cutaneous melanoma.
Setting
Tertiary university hospital.
Patients
A total of 515 patients with melanoma underwent a sentinel lymph node biopsy without evidence of metastatic disease between 1996 and 2008.
Main Outcome Measures
Time to recurrence and overall survival.
Results
Of 515 patients, 83 (16%) had a recurrence of melanoma at a median of 23 months during a median follow-up of 61 months (range, 1-154 months). Of these 83 patients, 21 had melanoma that metastasized in the studied nodal basin for an in-basin false-negative rate of 4.0%. Patients with recurrence had deeper primary lesions (mean thickness, 2.7 vs 1.8 mm; P<.01) that were more likely to be ulcerated (32.5% vs 13.5%; P<.001) than those without recurrence. The primary melanoma of patients with recurrence was more likely to be located in the head and neck region compared with all other locations combined (31.8% vs 11.7%; P<.001). Median survival following a recurrence was 21 months (range, 1-106 months). Favorable characteristics associated with lower risk of recurrence included younger age at diagnosis (mean, 49 vs 57 years) and female sex (9% vs 21% for males; P<.001).
Conclusion
Overall, recurrence of melanoma (16%) after a negative sentinel lymph node biopsy result was similar to that in previously reported studies with an in-basin false-negative rate of 4.0%. Lesions of the head and neck, the presence of ulceration, increasing Breslow thickness, older age, and male sex are associated with increased risk of recurrence, despite a negative sentinel lymph node biopsy result.
The American Cancer Society estimates that 76 250 new cases of melanoma will be diagnosed in the United States alone during 2012.1 The increasing incidence and prevalence of melanoma are in stark contrast to the overall decrease in the incidence rates of other cancers such as lung, prostate, breast, and colorectal cancer. Despite the increase in new cases, the percentage of patients with melanoma who have survived for 5 years has steadily increased compared with the percentages first recorded in 1975, from 82% to 93%, likely owing to earlier detection.2
Multiple indicators of overall survival with melanoma have been identified in previous studies, including the patient’s age,3 the patient’s sex,4 the Breslow thickness of the tumor,5 the presence of ulceration,6,7 and the tumor site.8 The strongest predictor for recurrence, however, is the status of the sentinel lymph node (SLN).6,9 Thus, the SLN biopsy (SLNB) has rapidly earned acceptance as the standard of care for most lesions thicker than 1 mm and for thin lesions with high-risk features such as ulceration or lymphovascular invasion.10,11
Because this is such an important prognostic factor, the reliability of the SLNB is key in determining prognosis and treatment, and it warrants further study, particularly for those who have a recurrence of melanoma after a negative SLNB result. Other studies12-14 have investigated local, regional, and/or in-transit recurrence after a negative SLNB result, but they are limited by a relatively short follow-up window. Unfortunately, patients with melanoma often experience a delayed recurrence; therefore, longer follow-up is warranted. The aim of our study was to evaluate the incidences of overall recurrence and of survival during long-term follow-up after a negative SLNB result and to compare our results with those at other institutions. We also sought to identify other factors associated with recurrence.
METHODS
A retrospective chart review of prospectively collected data was undertaken for all patients with melanoma who had undergone a successful SLNB at the University of Colorado Hospital in Aurora by 1 of 2 authors (N.W.P. and M.D.M.) between August 1996 and January 2008. The decision to undergo an SLNB was jointly made with input from the cutaneous oncology multidisciplinary team and was generally recommended for all patients who had a lesion with a Breslow thickness of greater than 1 mm or who had a thinner lesion with adverse features such as ulceration, a deep margin positive for melanoma, or lymphovascular invasion.
The study variables included age, sex, tumor site, Clark level of invasion, Breslow thickness of the tumor, histologic evidence of ulceration, lymphovascular invasion or regression, the presence or absence of mitoses, SLNB location and number of nodes removed, time to recurrence and location of recurrence, and survival time from diagnosis and recurrence. Recurrence was further categorized according to site into (1) local (within 2 cm of the original incision), (2) in-transit (>2 cm from the original incision but not included in the draining nodal basin), (3) regional (recurrence in the sample nodal basin), and (4) distant recurrence. The University of Colorado institutional review board approved our study.
Preoperative lymphoscintigraphy using a radio-labeled technetium 99m colloid injection and delayed imaging with marking of the location by the nuclear medicine radiologist was used for all patients. In addition, for selected patients, an intradermal injection of isosulfan or methylene blue dye at the excision site was given prior to the incision. Radioactive lymph nodes were removed until the basin included only nodes with counts less than 10% of the hottest node.15 Blue and clinically suspicious nodes were also removed. Finally, a wide local excision was performed with 1-cm margins for those lesions 1 mm or less in thickness and with 2-cm margins for those lesions greater than 1 mm. Cutaneous margins in cosmetically sensitive areas such as the head and neck were rarely modified at surgeon discretion, but all lesions in all regions were excised or re-excised to negative histologic margins. A partial or superficial parotidectomy was occasionally necessary to ensure adequate margins and sentinel node removal.
A pathologic evaluation of the SLNs was performed using the University of Colorado Melanoma protocol, which has evolved throughout the time period studied. Currently, the SLN is bisected, and the first level of the bisected lymph node is examined using a hematoxylin-eosin stain followed immediately by an HMB-45 immunohistochemical stain, which is then followed by removal of 250 μm of tissue and a second hematoxylin-eosin stain followed by a melan-A immunohistochemical stain. Another 250 μm of tissue is removed, and a third hematoxylin-eosin stain is followed by a tyrosinase stain.16 No sections of the bisected SLN were frozen. Patients with SLNs positive for melanoma or with regional recurrence were offered subsequent completion lymph node dissections (CLNDs).
Statistical analysis was undertaken using SAS version 9.2 (SAS Institute Inc). A 2-group t test (for mean values) or a Wilcoxon rank sum test (for median values) was used to compare continuous variables between the nonrecurrence and recurrence groups, and a χ2 test or a Fisher exact test was used to compare categorical variables between the 2 groups. Univariate and multivariable logistic regressions were used to assess the potential association between the outcome variable of recurrence and the demographic and clinical variables, as summarized in Table 1. Kaplan-Meier survival analysis was used to display survival functions. A log-rank test was used to test whether there was a significant difference between 2 survival curves. The level of significance was set at P≤.05.
Table 1.
Characteristics and Univariate Analysis of 520 Patents With Melanoma Who Underwent an SLNB Without Evidence of Metastatic Disease Between 1996 and 2008a
| Patients, No. (%) |
||||
|---|---|---|---|---|
| Characteristic | Negative SLNB Result (N = 520) |
No Recurrence (n = 437) |
Recurrence (n = 83) |
Univariate P Value |
| Age, mean (SD), y | 49.7 | 48.6 (14.2) | 56.8 (12.5) | <.001b |
| Male sex | 294 (56.5) | 231 (52.9) | 63 (75.9) | <.001c |
| Follow-up, median (range), mo | 61 | 62 (0-154) | 53 (4-152) | |
| Breslow thickness, mm | ||||
| Mean (range) | 1.9 (0.3-22.0) | 1.8 (0.5-22.0) | 2.7 (0.3-14.0) | <.01b |
| Median | 1.4 | 1.3 | 2.0 | <.001d |
| Left side of body | 256 (49.2) | 216 (49.4) | 40 (48.2) | .60c |
| Ulceration | 86 (16.5) | 59 (13.5) | 27 (32.5) | <.001c |
| Mitoses | 136 (26.2) | 112 (25.6) | 24 (28.9) | .40c |
| Lymphovascular invasion | 19 (3.7) | 16 (3.7) | 3 (3.6) | .99e |
| Regression | 15 (2.9) | 14 (3.2) | 1 (1.2) | .46e |
| No. of SLNs, mean (SD) | 1.95 | 2.09 (1.38) | 1.81 (0.89) | <.05b |
| No. of Non-SLNs, mean (SD) | 0.47 | 0.54 (1.36) | 0.41 (1.09) | |
| Type of melanoma | ||||
| Superficial spreading | 190 (36.5) | 167 (38.2) | 23 (27.7) | |
| Nodular | 68 (13.1) | 59 (13.5) | 9 (10.8) | |
| Lentiginous | 29 (5.6) | 20 (4.6) | 9 (10.8) | |
| Unknown/other | 233 (44.8) | 191 (43.7) | 42 (50.6) | |
| Location | ||||
| Head and neck | 110 (21.2) | 75 (17.2) | 35 (42.2) | <.001e |
| Trunk | 205 (39.4) | 181 (41.4) | 24 (28.9) | |
| Upper extremity | 82 (15.8) | 76 (17.4) | 6 (7.2) | |
| Lower extremity | 123 (23.7) | 105 (24.0) | 18 (21.7) | |
| Clark level | ||||
| I | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| II | 16 (3.1) | 14 (3.2) | 2 (2.4) | |
| III | 57 (11.0) | 48 (11.0) | 9 (10.8) | |
| IV | 417 (80.2) | 354 (81.0) | 63 (75.9) | |
| V | 25 (4.9) | 17(3.9) | 8 (9.6) | |
| SLNB location | ||||
| Head | 34 (6.5) | 21 (4.8) | 13 (15.7) | |
| Neck | 83 (1.6) | 60 (13.7) | 23 (27.7) | |
| Axilla | 210 (40.4) | 191 (43.7) | 19 (22.9) | |
| Groin | 150 (28.8) | 128 (29.3) | 22 (26.5) | |
| Multiple | 43 (8.3) | 37 (8.5) | 6 (7.2) | |
Abbreviations: SLN, sentinel lymph node; SLNB, SLN biopsy.
Of 515 patients, 5 (1.0%) had 2 separate lesions that were treated with additional but separate SLNBs at separate times, and both events were included in our study so that these 5 patients were counted twice for a total of 520 patients. On univariate analysis, male sex, older age at diagnosis, increasing Breslow thickness, histologic evidence of ulceration, and location in the head and neck region all demonstrated statistically significant P values of less than .05.
Determined by use of the t test.
Determined by use of the χ2 test.
Determined by use of the Wilcoxon rank sum test.
Determined by use of the Fisher exact test.
RESULTS
A total of 619 patients underwent a wide local excision and a successful SLNB at the University of Colorado between August 1996 and January 2008. Of these patients, 104 (16.8%) had a positive SLNB result and were excluded from our study, and 515 (83.2%) had a negative SLNB result and were included in our study. Of these 515 patients, 5 (1.0%) had 2 separate lesions that were treated with additional but separate SLNBs at separate times, and both events were included in our study so that these 5 patients were counted twice for a total of 520 patients. Forty-one of 660 patients (6.2%) had unsuccessful SLNBs and were not included in our study. The median follow-up time was 61.0 months (range, 0-154 months), and 294 patients (56.5%) were men. The median Breslow thickness was 1.4 mm, with 86 (16.5%) patients having lesions that exhibited ulceration on final pathologic examination. Detailed patient characteristics of the population are provided in Table 1.
Patients with a positive SLNB result were recommended to proceed with CLND. Of the 104 patients with a positive SLNB result, 85 (81.7%) actually underwent CLND, with additional positive nodes found in 17 of these patients (20.0%). Eighteen of the 104 patients (17.3%) did not undergo CLND because they either refused4 or were lost to follow-up.14
Of the 520 patients, 83 (16.0%) experienced recurrence after a negative SLNB result at a median of 23 months (range, 2-106 months) after diagnosis (Figure 1). Among these 83 patients, the initial documented site of recurrence was found to be local for 19 patients (22.9%) at a median of 14 months after SLNB, in-transit for 12 patients (14.5%) at 23 months, regional for 21 patients (25.3%) at 14 months, and distant for 26 patients (31.3%) at 30 months, with 5 patients (6.0%) experiencing a recurrence at an unrecorded site. Excluding local and in-transit recurrences, 52 of 520 patients (10.0%) with a negative SLNB result had a recurrence, and just 21 of 520 patients who underwent an SLNB experienced a recurrence in the sampled nodal basin for a false-negative rate of 4.0%.
Figure 1.
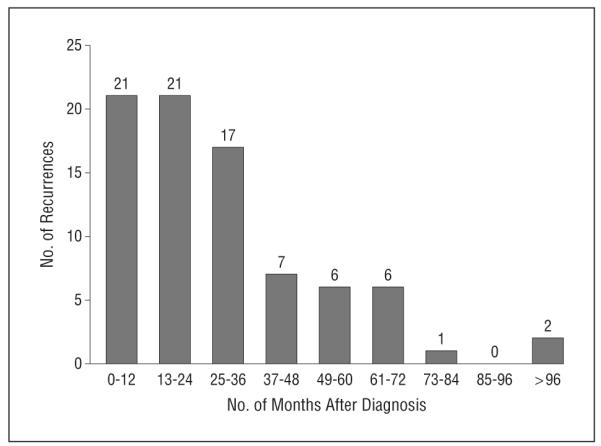
Number of recurrences by month after diagnosis. The median time to recurrence was 23 months, and the mean time to recurrence was 29.2 months. Two patients experienced recurrence more than 8 years after diagnosis, which affected the mean value (1 patient at 96 months and 1 patient at 106 months).
Patients with a regional recurrence were recommended to proceed with CLND. Fourteen of the 21 patients with a regional recurrence (66.7%) underwent CLND, with additional lymph nodes positive for melanoma in 10 of the 14 patients (71.4%). This was significantly more often than for the patients with a positive SLNB result (71.4% vs 16.3%; P < .001, determined by use of the Fisher exact test). The remaining 7 patients either declined CLND4 or were lost to follow-up.3
On univariate analysis, the patients who were more likely to have any recurrence after a negative SLNB result were men (75.9%; P < .001), had deeper lesions (mean Breslow thickness, 2.7 vs 1.8 mm; P < .01, determined by use of the 2-group t test), and had fewer SLNs evaluated (mean number, 1.81 vs 2.09; P < .05) than women. In addition, lesions located in the head and neck region were more likely to recur, accounting for 42.2% (P < .001, determined by use of the Fisher exact test) of all recurrences (Figure 2). Using the American Joint Council on Cancer 2009 melanoma of the skin staging criteria, patients without recurrence were more likely to have T1 lesions (136 patients without recurrence vs 10 patients with; P < .001). A comparison by T category is provided in Table 2.
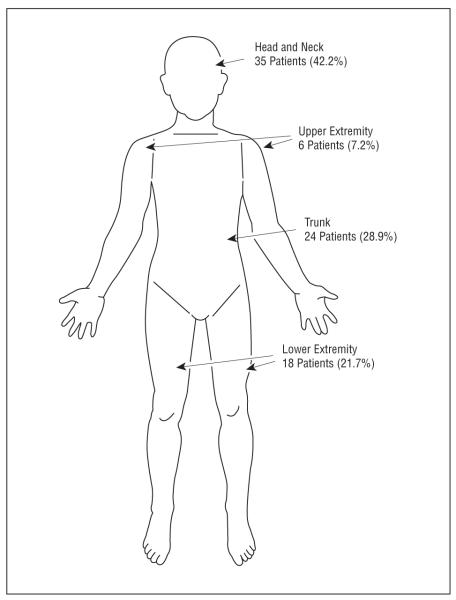
Figure 2.
Recurrence by site of primary melanoma in 83 patients. The majority of patients with recurrences had primary lesions located in the head and neck region, followed by the trunk. Primary lesions in the upper extremity accounted for the smallest portion of the recurrences.
Table 2.
Lesions by T Category of 520 Patents With Melanoma Who Underwent an SLNB Without Evidence of Metastatic Disease Between 1996 and 2008a
| Patients, No. |
||||
|---|---|---|---|---|
| Categoryb | Negative SLNB Result (N = 520) |
No Recurrence (n = 437) |
Recurrence (n = 83) |
P Valuec |
| T1 | 146 | 136 | 10 | <.001 |
| T2 | 225 | 193 | 32 | .40 |
| T3 | 111 | 86 | 25 | .04 |
| T4 | 35 | 20 | 15 | <.001 |
| Unknown | 3 | 2 | 1 | NA |
Abbreviations: NA, not applicable; SLNB, SLN biopsy.
Of 515 patients, 5 (1.0%) had 2 separate lesions that were treated with additional but separate SLNBs at separate times, and both events were included in our study so that these 5 patients were counted twice for a total of 520 patients.
Using the American Joint Council on Cancer 2009 melanoma of the skin staging criteria.
Determined by use of the Fisher exact test.
Superficial spreading was the most prevalent type of melanoma in all groups. The presence of ulceration was found significantly more often in patients with a negative SLNB result who had recurrent lesions than in patients with a negative SLNB result who did not have recurrent lesions (32.5% vs 13.5%; P < .001). Clark level, mitoses, lymphovascular invasion, and regression were not predictive of recurrence in this analysis.
The variables described were further examined using multivariate analysis, and all except sex remained significant. Older age at diagnosis, increasing Breslow thickness of the primary lesion, the presence of ulceration, and lesions located in the head and neck region continued to be more prevalent in patients who experienced a recurrence after a negative SLNB result than in patients who did not experience a recurrence after a negative SLNB result (Table 3).
Table 3.
Multivariate Analysis of Factors Predictive of Recurrencea
| Risk Factor | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Ageb | 1.04 (1.02-1.06) | <.001 |
| Breslow thicknessb | 1.16 (1.04-1.29) | .008 |
| Ulceration (yes vs no) | 2.73 (1.44-5.16) | .001 |
| Head and neck region (yes vs other) |
3.02 (1.76-5.18) | <.001 |
Male sex is no longer statistically significant.
Odds ratios for age and Breslow thickness refer to increasing age and increasing Breslow thickness, respectively.
Lastly, a survival analysis was undertaken to determine the effect on survival of recurrence after a negative SLNB result. Of the 83 patients with recurrence after a negative SLNB result, 40 (48.2%) died with a median survival of 15.5 months (range, 1-73 months) after recurrence. Figure 3 shows the overall survival of the patients with a negative SLNB result, both those with and those without recurrence. This demonstrates that patients with a negative SLNB result who experienced a recurrence had a significantly decreased 5-year overall survival probability (68% [95% CI, 59%-76%]) compared with patients with a negative SLNB result who did not experience a recurrence (98% [95% CI, 96%-99%]). The overall 5-year survival probability in our study is 91% for all patients who tested negative for melanoma by use of an SLNB.
Figure 3.
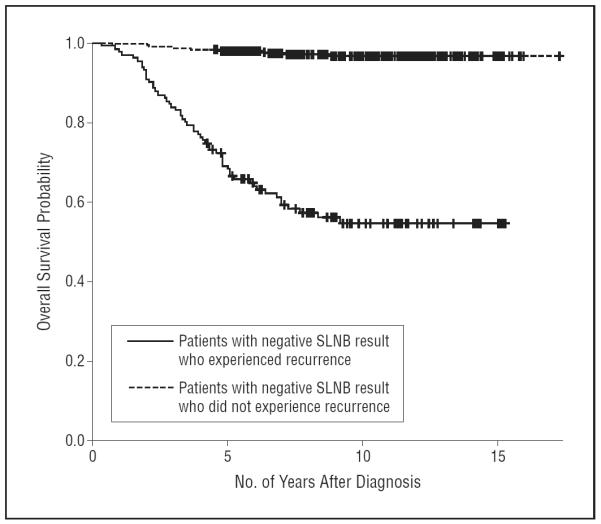
Overall survival probability for patients with and patients without recurrence. Kaplan-Meier overall survival curves show the number of months after diagnosis of survival. Patients with a negative sentinel lymph node biopsy (SLNB) result who experienced recurrence had an overall 5-year survival probability of 68%, and patients with a negative SLNB result who did not experience recurrence had an overall 5-year survival probability of 98%. The overall 5-year survival probability for patients with a negative SLNB result is 91%.
Among patients with a recurrence, 8 of 19 patients with a local recurrence (42.1%) died during the study period, 6 of 12 patients with an in-transit recurrence (50.0%) died, 11 of 21 patients with a regional recurrence (52.4%) died, and 13 of 26 with a distant recurrence (50.0%) died. There were 5 patients with an unknown location of recurrence, 2 of whom died (40.0%). The limited data do suggest that there is a significant difference in survival in terms of location of the initially detected distant recurrence (P < .05, determined by log-rank test): 4 of 8 patients with recurrence in the lung (50.0%) died, 2 patients with recurrence in the liver died, 2 of 5 patients with recurrence in the brain (40.0%) died, 1 patient with recurrence in the gastrointestinal tract died, and 6 of 8 patients with multiple recurrences (75.0%) died. Of the 2 patients with other locations of recurrence, 1 (50.0%) died. A log-rank test that did not include the patients with unknown locations of recurrence indicates that there is no statistically significant difference in overall survival from the time of recurrence among patients with different sites of recurrence (P = .42).
COMMENT
Numerous studies6,8 have confirmed the unequivocal prognostic value of an SLNB in cutaneous melanoma. In fact, a negative SLNB result portends a good outcome with a low risk of recurrence and an overall 5-year survival probability of 91% in our study. This test is not perfect, however, and false-negative results are possible but thought to be uncommon.14 We sought to more clearly define the factors that predict which patients are at risk for recurrence of melanoma after a negative SLNB result. Older age at diagnosis, deeper lesions, the presence of ulceration on histologic examination, and location in the head and neck region were all more common in the patients with recurrence.
The false-negative rate of 4.0% is consistent with previous studies13,14,17 and is defined herein as the incidence of recurrence in the previously biopsied draining nodal basin. However, some patients will develop distant metastases without evidence of metastases within the studied nodal basin.14 These patients, who cannot as yet be defined, would not benefit from the information gained by an SLNB. It is the patients with recurrence in the studied basin that are most likely to benefit from an improved understanding and sampling technique for an SLNB.
In our study, the most common anatomical sites of the primary lesion for those patients with recurrence after a negative SLNB result were in the head and neck region. Previous studies have also documented this, although the reasons are not entirely clear.18 Accuracy may be compromised by ambiguity or multiplicity in the local lymphatic drainage patterns, as well as in the techniques of injection and the “shine through” from radioactivity around the primary site. However, the possibility that melanoma of the head and neck possesses a more aggressive biologic makeup has yet to be excluded.
The mechanism behind the association between advanced age and increased risk of recurrence is unclear but may be due to age-related lymphatic dysfunction resulting in the delayed distribution of tumor cells to nodes at the time of surgery.19 This hypothesis suggests that older patients may be at increased risk of false-negative results. Deeper lesions were also associated with an increased risk of recurrence, consistent with the findings of previous studies.4,5,13,14 An increased tumor burden logically increases the distribution of cells and may result in other microscopically positive nodes that are not removed owing to low radiotracer counts at surgery.
The single microscopic feature that was predictive of recurrence was ulceration. Classically, ulceration is thought to represent a more aggressive lesion. Other studies have suggested the presence of lymphovascular invasion,20,21 regression, and/or increased mitotic activity as additional evidence of a more aggressive lesion, but definitive data are not yet available.22,23 In fact, a number of histologic and other factors were studied here but did not reach statistical significance. However, it is important to note that 54% of the pathology reports were missing at least 1 of the studied factors in their pathologic analysis, which significantly limits the power of any conclusions in this area.
The importance of long-term follow-up for these patients is emphasized by the fact that the median time to recurrence occurred almost 2 years (23 months) after diagnosis. Patients with recurrence survived, on average, another 21 months after recurrence, resulting in a 5-year overall survival probability of 64%, which is remarkably similar to that for patients with stage III disease, who had an average 5-year survival probability of 63% (67% for nodal micrometastases only).24 Because this subgroup behaves similarly to patients with a positive SLNB result, an important question is whether this is a technical failure to locate the SLN or a more aggressive melanoma subtype.
When combined with the survival probability of patients without recurrence, the 5-year overall survival probability increases to 91%, which is consistent with other published data on survival.13,14 Because many of these patients have prolonged disease-free and overall survival, the optimal, final “postoperative” visit has yet to be determined. Using data from our study, a follow-up period of just 5 years would have missed 10.8% (9 of 83 patients) of recurrences.
Interestingly, the initial location of recurrence (local, in-transit, regional, or distant) did not significantly alter prognosis. It is fair to say that any recurrence, regardless of initial site, is a poor prognostic sign; however, the supposition that a local or in-transit metastasis was caught at an earlier time and that another resection may yet result in improved survival did not hold up in our analysis. Although the overall number of patients is low, among patients with distant metastasis, the location of the distant metastasis was significant: patients with gastrointestinal, liver, and/or multiple metastases tend to have a reduced survival probability.
In conclusion, our study confirms a low in-basin false-negative rate for SLNB for patients with melanoma. In addition, several characteristics of the lesions were predictive of recurrence after a negative SLNB result. Specifically, lesions of the head and neck, the presence of ulceration, increasing Breslow thickness, older age, and male sex were all associated with an increased risk of recurrence after a negative SLNB result. Long-term follow-up for this group of patients is necessary owing to the high proportion of patients who may develop delayed metastasis. Finally, the location of recurrence does not change the poor prognosis of the recurrence, in and of itself.
Footnotes
Author Contributions: Dr E. L. Jones had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E. L. Jones and McCarter. Acquisition of data: E. L. Jones, T. S. Jones, Pearlman, Stovall, Gonzalez, Lewis, Robinson, and McCarter. Analysis and interpretation of data: E. L. Jones, T. S. Jones, Pearlman, Gao, Gajdos, Kounalakis, Gonzalez, and McCarter. Drafting of the manuscript: E. L. Jones and T. S. Jones. Critical revision of the manuscript for important intellectual content: E. L. Jones, Pearlman, Gao, Stovall, Gajdos, Kounalakis, Gonzalez, Lewis, Robinson, and McCarter. Statistical analysis: E. L. Jones and Gao. Administrative, technical, and material support: T. S. Jones, Stovall, Robinson, and McCarter. Study supervision: Pearlman, Gajdos, Kounalakis, Gonzalez, and McCarter.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Cancer facts and figures 2005. American Cancer Society; [Accessed April 27, 2012]. website. http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-factsfigures-2005. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Kretschmer L, Starz H, Thoms KM, et al. Age as a key factor influencing metastasizing patterns and disease-specific survival after sentinel lymph node biopsy for cutaneous melanoma. Int J Cancer. 2011;129(6):1435–1442. doi: 10.1002/ijc.25747. [DOI] [PubMed] [Google Scholar]
- 4.Scoggins CR, Ross MI, Reintgen DS, et al. Sunbelt Melanoma Trial. Gender-related differences in outcome for melanoma patients. Ann Surg. 2006;243(5):693–698. doi: 10.1097/01.sla.0000216771.81362.6b. discussion 698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA. A multifactorial analysis of melanoma: II, prognostic factors in patients with stage I (localized) melanoma. Surgery. 1979;86(2):343–351. [PubMed] [Google Scholar]
- 6.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 7.McMasters KM, Noyes RD, Reintgen DS, et al. Sunbelt Melanoma Trial. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004;86(4):212–223. doi: 10.1002/jso.20084. [DOI] [PubMed] [Google Scholar]
- 8.Kienstra MA, Padhya TA. Head and neck melanoma. Cancer Control. 2005;12(4):242–247. doi: 10.1177/107327480501200406. [DOI] [PubMed] [Google Scholar]
- 9.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976–983. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 10.Mays MP, Martin RC, Burton A, et al. Should all patients with melanoma between 1 and 2 mm Breslow thickness undergo sentinel lymph node biopsy? Cancer. 2010;116(6):1535–1544. doi: 10.1002/cncr.24895. [DOI] [PubMed] [Google Scholar]
- 11.Scoggins CR, Bowen AL, Martin RC, II, et al. Prognostic information from sentinel lymph node biopsy in patients with thick melanoma. Arch Surg. 2010;145(7):622–627. doi: 10.1001/archsurg.2010.115. [DOI] [PubMed] [Google Scholar]
- 12.Carlson GW, Page AJ, Cohen C, et al. Regional recurrence after negative sentinel lymph node biopsy for melanoma. Ann Surg. 2008;248(3):378–386. doi: 10.1097/SLA.0b013e3181855718. [DOI] [PubMed] [Google Scholar]
- 13.Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16(6):2253–2260. doi: 10.1200/JCO.1998.16.6.2253. [DOI] [PubMed] [Google Scholar]
- 14.Scoggins CR, Martin RC, Ross MI, et al. Factors associated with false-negative sentinel lymph node biopsy in melanoma patients. Ann Surg Oncol. 2010;17(3):709–717. doi: 10.1245/s10434-009-0858-x. [DOI] [PubMed] [Google Scholar]
- 15.Carlson GW, Murray DR, Thourani V, Hestley A, Cohen C. The definition of the sentinel lymph node in melanoma based on radioactive counts. Ann Surg Oncol. 2002;9(9):929–933. doi: 10.1007/BF02557533. [DOI] [PubMed] [Google Scholar]
- 16.Spanknebel K, Coit DG, Bieligk SC, Gonen M, Rosai J, Klimstra DS. Characterization of micrometastatic disease in melanoma sentinel lymph nodes by enhanced pathology: recommendations for standardizing pathologic analysis. Am J Surg Pathol. 2005;29(3):305–317. doi: 10.1097/01.pas.0000152134.36030.b7. [DOI] [PubMed] [Google Scholar]
- 17.Morton DL, Cochran AJ, Thompson JF, et al. Multicenter Selective Lymphadenectomy Trial Group Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–311. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 311-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saltman BE, Ganly I, Patel SG, et al. Prognostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32(12):1686–1692. doi: 10.1002/hed.21390. [DOI] [PubMed] [Google Scholar]
- 19.Conway WC, Faries MB, Nicholl MB, et al. Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol. 2009;16(6):1548–1552. doi: 10.1245/s10434-009-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clary BM, Brady MS, Lewis JJ, Coit DG. Sentinel lymph node biopsy in the management of patients with primary cutaneous melanoma: reviewof a large single-institutional experience with an emphasis on recurrence. Ann Surg. 2001;233(2):250–258. doi: 10.1097/00000658-200102000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao C, Wong SL, Ross MI, et al. Sunbelt Melanoma Trial Group Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg. 2002;184(6):520–524. doi: 10.1016/s0002-9610(02)01102-9. discussion 525. [DOI] [PubMed] [Google Scholar]
- 22.Roach BA, Burton AL, Mays MP, et al. Does mitotic rate predict sentinel lymph node metastasis or survival in patients with intermediate and thick melanoma? Am J Surg. 2010;200(6):759–763. doi: 10.1016/j.amjsurg.2010.07.037. discussion 763-764. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Gershenwald JE, Soong SJ, Thompson JF. Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol. 2011;104(4):379–385. doi: 10.1002/jso.21876. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]