Abstract
Normal aging is associated with a gradual decline in executive functions such as set-shifting, inhibition, and updating, along with a progressive decline of neurotransmitter systems including the dopamine system. Modulation from the dopamine system is thought to be critical for the gating of information during working memory. Given the known relationships between executive aging, cognition, and dopamine, this study aims to explore the neurobiology underlying age-related changes in working memory updating using fMRI with healthy subjects from across the adult age spectrum. Our results indicate that older age is associated with poorer performance, reduced meso-cortico-striatal activation, and reduced functional coupling between the caudate and the VLPFC during the updating task. Additionally, caudate activation is associated with improved accuracy and VLPFC activation with faster reaction times in the full sample. Thus, older subjects’ under-recruitment of and reduced functional coupling between these regions may specifically underlie age-related changes in working memory updating. These results are consistent with computational models of executive cognition and dopamine-mediated age-related cognitive decline.
Keywords: working memory, aging, fMRI, functional connectivity, caudate, prefrontal cortex
1. Introduction
Working memory (WM) commonly refers to a cognitive system of integrated sub-processes that enable online storage and manipulation of information required for more complex tasks such as learning, reasoning, and decision-making (Baddeley, 1992). The central executive, or “attentional-controlling” sub-component of Baddeley’s WM model is considered responsible for functions such as updating, set-shifting, and inhibition (Baddeley, 1992,Miyake, et al., 2000), all of which show a gradual decline with older age (Braver and Barch, 2002,Clarys, et al., 2009,Fisk and Sharp, 2004). Computational models have posited that central executive functions are primarily mediated by dopaminergic modulation of cortico-striatal network activity (Braver and Barch, 2002,Frank, et al., 2001,Hazy, et al., 2006,O’Reilly and Frank, 2006), and it is hypothesized that neuromodulatory insufficiency may play a central role in age-related cognitive decline, perhaps by altering the integrity of this meso-cortico-striatal functional network. Such changes to the meso-cortico-striatal system would likely be reflected by activation and functional connectivity changes during tasks probing the central executive component of WM. The present study therefore aims to investigate the effects of normal aging on meso-cortico-striatal activation and connectivity during WM updating using an event-related functional magnetic resonance imaging (fMRI) task capable of parsing activation from WM executive sub-component processes (e.g., updating, maintenance, and overwriting), using cognitive subtraction logic (Posner, et al., 1988, Smith and Jonides, 1997).
fMRI studies have previously demonstrated that healthy older subjects both under-recruit task-relevant brain regions and recruit additional cortical resources compared to younger subjects, showing, for example, less cortical asymmetry during both simple motor and higher cognitive tasks (Cabeza, 2002,Mattay, et al., 2002,Reuter-Lorenz, et al., 2000). Under-recruitment is thought to reflect a less efficient engagement of specialized task-specific networks important for performance (Goh, 2011, Peelle, et al., 2010). Recruitment of additional resources in older individuals may be an adaptive strategy to maintain performance in the face of decline in the function of canonical neural circuits and may therefore represent evidence of compensation (Cabeza, 2002,Davis, et al., 2008,Park and Reuter-Lorenz, 2009,Rajah and D’Esposito, 2005). Alternatively, according to the dedifferentiation hypothesis, as brain responses become less specialized with increasing age, they may respond more similarly across a range of cognitive tasks and demonstrate engagement of brain areas not normally active in a particular task in younger subjects (Goh, 2011).
Given the well-established role of the prefrontal cortex (PFC) in WM (Goldman-Rakic, 1995), age-related WM deficits have frequently been attributed to structural and functional changes to the PFC, but the directionality of activation differences seems to depend on task demands, behavioral performance, and capacity constraints (Emery, et al., 2008,Mattay, et al., 2006,Nagel, et al., 2009,Nyberg, et al., 2009,Rajah and D’Esposito, 2005,Rypma, et al., 2007).
In addition to specific regional defects, neurotransmitter system decline, especially dopamine, has been proposed to play a causal role in age-related cognitive changes and their associated neurophysiological phenotypes. Converging evidence indicates that dopamine concentration, transporter availability, and receptor density decline at a rate of about 10% per decade from early to later adulthood (Backman, et al., 2000,Backman, et al., 2006,Hedden and Gabrieli, 2004). While a causal link between dopamine and age-related cognitive decline is difficult to explicitly establish, the evidence for its critical role in cognition is substantial. Beyond the aging literature, neuropsychiatric disorders for which dopaminergic system alterations have been implicated (including Parkinson’s disease, Huntington’s disease, and schizophrenia) also profoundly impair executive cognition (Cropley, et al., 2006). Additionally, dopamine system genetic variation, as in the Val158Met Catechol-O-Methyltransferase (COMT) polymorphism and the dopamine transporter 3′ variable nucleotide tandem repeat (DAT 3′ VNTR), alters brain activation during cognitive executive functions (Lindenberger, et al., 2008,Sambataro, et al., 2009,Tan, et al., 2007).
In computational models of executive control, dopamine acts tonically to bias cortical representations of the current WM context and phasically to open the “gate” to allow motivationally salient or task-relevant information to gain access to on-line WM representations (Braver and Barch, 2002,Frank, et al., 2001,Gruber, et al., 2006,Hazy, et al., 2006,O’Reilly and Frank, 2006). More specifically, modulatory signals from the dopaminergic midbrain, namely the substantia nigra/ventral tegmental area (SN/VTA), tune gating mechanisms in the striatum to mediate prefrontal cortical activity involved in the maintenance and manipulation of on-line WM representations (Hazy, et al. 2006). A task that requires an individual to update some specific information while maintaining other specific information in the WM store presents a challenge to these computational models, which is met by the existence of differentially activated parallel fronto-striatal loops that allow access to the current WM representation, and the central executive learns when to update versus maintain by dopaminergic reinforcement (Gruber 2006; Hazy, et al. 2006).
Using a novel WM updating fMRI task, we have previously shown that selective WM updating preferentially engages the meso-cortico-striatal system (including the SN/VTA, dorsal striatum, and lateral prefrontal cortex) compared to simple WM maintenance and overwriting (Murty, et al. 2011). These findings support the previously mentioned computational models of dopamine-mediated network activity underlying WM updating, during which the central executive selectively determines when to update the WM store with incoming new information (Frank, et al., 2001,Hazy, et al., 2006,O’Reilly and Frank, 2006).
In models of cognitive aging attributed to dopamine system dysfunction, a partially blocked dopaminergic “gate” and reduced tonic cortical stabilization of WM representations make information less reliable and more susceptible to interference during tasks involving executive cognitive control (Braver and Barch, 2002). However, the effects of age on brain activation and functional connectivity during a task isolating selective WM updating have not yet been studied. Because the task that we use in the present study has been validated to assess meso-cortico-striatal networks (Murty, et al. 2011), we believe that it may serve as a useful tool for investigating age-related changes to this network subserving executive functions. While age-related changes to this system may be mediated by dopamine system decline, the present fMRI study investigates the integrity of the system specifically in terms of functional activation, connectivity, and performance. The updating task is thus applied to examine how aging affects this ubiquitous executive sub-process of WM, involving the selective gating of information into the on-line WM store.
2. Materials and Methods
2.1 Subjects
Participants were recruited through the National Institutes of Health normal volunteer office, and the protocol was approved by the National Institute of Mental Health Institutional Review Board. Healthy volunteers with normal or corrected-to-normal visual acuity were studied. Exclusion criteria included past history or presence of any medical, neurological, or psychiatric disorders according to DSM-IV (following a Structured Clinical Interview, SCID-IV) (First, 1996), past head trauma with loss of consciousness, and drug treatment (except birth control pills in 1 young woman and hormonal substitution therapy in 1 postmenopausal woman and 2 subjects with hypothyroidism). Older subjects underwent a thorough neuropsychological assessment in order to evaluate cognitive status and to exclude gross cognitive decline (Mini Mental State Examination>28, Clinical Dementia Rating=0). The sample included data from 47 healthy volunteers (27 males, 20 females; age ± SD: 37.6 ± 16.1, range: 20-71 yrs; IQ ± SD: 112.6 ± 7.5) selected from a larger sample of subjects who passed imaging quality control measures to obtain a sample that did not show correlation between age and variables including IQ, handedness, education, etc. The distribution of young (<35), middle (35-55), and older (>55) aged subjects was skewed towards younger ages with 29 young, 7 middle, and 11 older subjects. Data from 28 subjects (<45 years old) were reported previously by Murty, et al. (2011). Handedness was assessed using the Edinburgh Questionnaire (Oldfield, 1971), and age did not correlate with handedness scores (mean score ± SD: 87.2 ± 26.3). All subjects gave written informed consent after the procedure was fully explained to them. A subset of this sample was used in a supplementary two-sample analysis comparing younger and older subjects (see supplementary materials for details).
2.2 Cognitive task
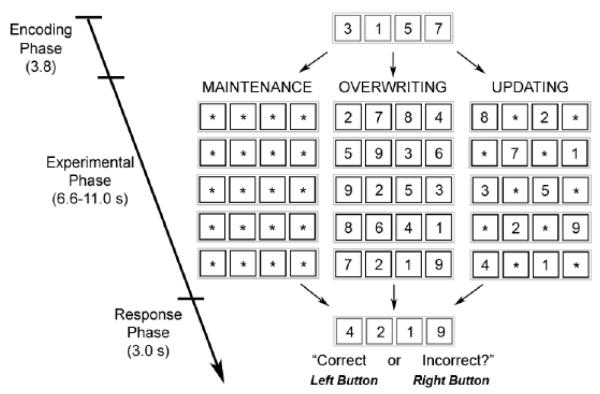
Subjects performed the Updating WM task (Figure 1) (Murty, et al. 2011). The task was designed to isolate the selective updating component of WM by comparing brain activation during Maintenance (MAI), Overwriting (OVR) and Updating (UPD) trial types. Contrasts between these conditions employ the logic of cognitive subtraction to isolate the neural underpinnings of cognitive processes involved in one condition but not the other (Posner, M.I., et al., 1988, Smith, E.E., Jonides, J., 1997).
Figure 1. Schematic diagram of cognitive task.
Each trial began with an encoding phase during which participants had to encode four digits. During the experimental phase, subjects maintained previously encoded digits when presented with asterisks and encoded new digits when presented with one. Only asterisks were shown during maintenance trials, only digits were shown during overwriting trials, and 1-3 digits were shown during updating trials with the rest of the boxes being filled with asterisks. During the response phase, subjects indicated via button press whether or not the digit string presented matched his/her internal stored working memory representation.
The task consisted of 45 trials (9 MAI, 9 OVR, and 27 UPD), presented over 3 runs. Each trial consisted of encoding, experimental, and response phases. Trial order was pseudo-randomized across each run. Between trials, a fixation crosshair was presented for inter-trial intervals of 3.1 ± 1.2 seconds.
During the encoding phase, subjects viewed four digits in blue boxes (3.8 seconds). Next, during the experimental phase, participants were shown 3 to 5 sequential presentations of 4 black boxes (shown for 2.0 seconds each) that contained digits and/or asterisks (6.6-11.2 seconds). If a box contained an asterisk, participants would continue to store the number previously shown in that position, but if the box contained a new digit, they would disregard the previous number and encode the new digit. Finally, during the response phase, participants were shown red boxes containing four digits (3.0 seconds), and they were instructed to respond with a right button-press if this digit set matched the set they were currently maintaining, or a left button-press if it did not. The digits displayed in the response phase of the task represented correct matches in half of trials in all conditions. For incorrect matches, the final digit string was incorrect by only one digit.
Experimental conditions were manipulated by changing the ratio of asterisks to digits. During MAI trials, black boxes contained only asterisks, so participants maintained the original digit set from the encoding phase over 5 serial presentations of black boxes with asterisks until the response phase (11.2 seconds). During OVR trials, black boxes presented over 3 to 5 serial presentations (6.6-11.2 seconds) contained no asterisks and only new digits, so subjects repeatedly cleared the digit set they had been maintaining, replacing it with a newly encoded set. The number of serial presentations varied during this trial type to ensure that subjects attended to each number set (and not just the final set before the response phase). During UPD trials, 5 serial presentations of black boxes (11.2 seconds) contained either 1, 2, or 3 digits, with the rest of the boxes containing asterisks. For each trial, the number of updates required at each black box presentation was held constant (9 trials each of 1, 2, and 3-digit UPD trial-types). Subjects were thus asked to maintain previously seen digits designated by asterisks while clearing and overwriting those designated by new digits,
While MAI, OVR, and UPD trials each involve some aspects of WM, UPD importantly requires selective and specific updating of the WM representation depending on the placement of asterisks and new digits, which indicate which information is to be maintained and which is to be updated. OVR trials also involve WM updating, but this updating is non-selective and non-specific, as the entire representation is cleared and a new one is encoded with each new stimulus presentation. Following cognitive subtraction logic (Posner, M.I., et al., 1988, Smith, E.E., Jonides, J., 1997), an fMRI contrast modeling the difference between the experimental phases of UPD and OVR trials should thus capture activation related to the selective and specific gating of information to the WM store, an executive function hypothesized to depend on meso-cortico-striatal signalling.
2.3 MRI acquisition
Subjects were scanned on a GE Signa (Milwauke, WI) 3-Tesla scanner. A gradient echo blood oxygenation level-dependent (BOLD-EPI) pulse sequence was used to acquire 160 images/run. Each functional image consisted of 26 interleaved 4-mm thick axial slices (TR/TE = 2000/28 ms; matrix = 64×64 mm; gap = 1 mm; field of view = 24 cm; flip angle = 90°). Participants were scanned while performing 3 runs (5 minutes and 20 seconds each) of the task described above. Stimuli were presented using Presentation software via a back-projection system, and the button-press responses were recorded through a fiber optic response box, which allowed measurement of accuracy (% correct of decisions) and reaction time (ms) for each trial. The first four scans were acquired to allow signal saturation and were not included in analyses.
2.4 Data analysis
2.4.1 Behavioral data
Descriptive statistics for accuracy (%) and reaction time (ms) were calculated for each condition. The effect of task condition (MAI, OVR, UPD) on performance was tested using a repeated-measures ANOVA with task condition as a within-subjects factor in order to assess relative difficulty between conditions. Bivariate Pearson correlations were also performed to investigate the relationships between age (modeled as a continuous variable throughout this study) and behavioral data for each condition, and between-condition differences in age vs. performance correlations were also assessed using the Fisher’s r to z transformation. Behavioral statistical analyses were conducted using SPSS 13.0 set to a threshold of p< 0.05.
2.4.2 Functional Imaging Data
Individual subjects’ imaging data were preprocessed using SPM5 (www.fil.ion.ucl.acuk/spm) with slice timing, realignment, normalization to an MNI template using a 4th degree B-spline interpolation, and smoothing using an isotropic 10 mm3 Full-Width-Half-Maximum kernel. Individual data-sets were carefully screened via quality inspection for image and ghosting artifacts, low signal-to-noise ratios, high signal variance across brain images within the time series, excessive head motion (> 3 mm translation or 1.5 degrees rotation), and lack of motor activation associated with button press (assessed by subjects’ retrieval>baseline contrasts).
First-level individual statistical parametric maps (SPMs) were constructed using a general linear model (GLM) across all three runs; regressors were modeled as mini-boxcars convolved with the canonical hemodynamic response function, normalized to the global signal across the whole brain and high-pass filtered with a cut-off frequency of 1/128 Hz to remove the effects of scanner signal drifts. For each individual SPM, regressors of interest were modeled for the experimental phase during correct OVR, MAI, and UPD trials separately. Data from incorrect trials were modeled separately and not included in analyses. Other regressors of no interest included 6 head motion parameters and the encoding and retrieval phases of each condition as the current study explored to isolate the cognitive operations engaged during working memory rather than simple encoding or decision-criteria. Using the GLM, individual SPMs were generated for the following t-contrasts: UPD>baseline, MAI>baseline, OVR>baseline, UPD>OVR, UPD>MAI, encoding>baseline, and retrieval>baseline. The implicit baseline (used for all but UPD>OVR and UPD>MAI contrasts) included all time points not explicitly modeled by our regressors, namely the inter-trial intervals during which fixation crosshairs were presented.
Second level random-effects analyses were performed on the UPD> baseline, MAI> baseline, and OVR> baseline, and UPD>OVR contrasts separately. As previously discussed, UPD>OVR served as the main contrast of interest in order to isolate the executive gating component of UPD by subtracting activation due to non-selective and non-specific WM clearing and encoding, attention, and mental rehearsal.
The effect of age on activation during UPD>OVR was investigated by entering subjects’ ages into a regression with their contrast images. Supplemental analyses assessing age-related effects in UPD and OVR compared to baseline were similarly performed. The relationships between task performance and brain activation in UPD>OVR were also investigated; the percent difference in accuracy and reaction time between UPD and OVR conditions were calculated for each subject [(UPD-OVR)/(UPD+OVR)], and these scaled performance values were entered as covariates of interest in regression analyses with subjects’ UPD>OVR contrasts. Supporting supplementary two-sample analyses also assessed the effect of age using the UPD>OVR contrast (see supplementary materials for details).
Because of our a priori hypotheses regarding the involvement of the dorsal striatum and the prefrontal cortex in this task (Murty, et al. 2011; Braver and Barch 2002), and because clusters of activation within the caudate and VLPFC were found to correlate with both age and performance in this sample (see results), we assessed the functional connectivity of these regions. Anatomical masks were used to derive time courses of activation within right and left caudate and right and left VLPFC separately (see Statistics section for description of these regions) during the UPD condition, and a beta series analysis was used to construct whole-brain maps of seed-to-voxel time activity correlations for the four seed regions for each subject (Rissman, et al., 2004). This technique has been used to model functional connectivity during distinct cognitive stages of event-related fMRI tasks by calculating a parameter estimate (beta value) for each stage of each trial and then sorting these values according to the stage from which they were derived to yield a “beta series.” In the present analysis, each seed’s beta series was estimated from the median parameter estimates of five contiguous voxels with the most task-related activity (assessed by an F test with a p threshold of 0.05) for the experimental phase of UPD trials. Each seed’s beta series was then assessed for correlations with that of every other voxel in the brain, and the resulting seed-to-voxel correlation data underwent Fisher’s r to z transformations to generate whole brain z-maps of positive coupling with the seed regions of interest during UPD for each subject. Because task-induced deactivation was not the subject of this study and was not assessed with a univariate analysis, the functional connectivity analysis was restricted to positive coupling, as negative coupling with these task-relevant ROIs would yield deactivated anticorrelated results.
Individual SPMs of functional connectivity were subsequently entered into second level random-effects analyses using one-sample t-tests to assess group-level functional coupling with each region of interest during UPD. Age was then entered as a regressor of interest with subjects’ functional connectivity maps in order to assess age-related changes in functional coupling. Performance-related differences in functional connectivity were also assessed by similarly entering subjects’ UPD accuracy and RT as regressors of interest in separate analyses.
2.4.3 Statistics
The statistical threshold for all second-level analyses was set at p<0.05, false discovery rate (FDR) corrected(Genovese, et al., 2002). Age and performance regression maps were masked by second-level main effect maps set to threshold of p<0.05, uncorrected, in order to limit these analyses to task-related activations. We performed Regions of Interest (ROI) analyses using small volume corrections for the bilateral caudate, midbrain substantia nigra/ventral tegmental area (SN/VTA), dorso-lateral prefrontal cortex (DLPFC), and ventro-lateral prefrontal cortex (VLPFC) because of these regions’ known involvement in meso-cortico-striatal circuitry proposed to underlie cognitive control and the executive functions of WM (Braver and Barch, 2002,Frank, et al., 2001,Hazy, et al., 2006,O’Reilly and Frank, 2006). Results-independent masks of these regions were constructed using structural labels from the WFU PICKATLAS tool based on stated a priori hypotheses (Supplementary Figure 1). The caudate ROI was formed by the addition of caudate head and caudate body labels. The SN/VTA ROI was made by dilating the substantia nigra label by 1 to also include the VTA. The DLPFC ROI included lateral portions of Brodmann Areas 9 and 46, constructed in WFU PICKATLAS by adding dilated Brodmann Areas 9 and 46 and subtracting out the medial portions. The VLPFC ROI was similarly made by adding dilated Brodmann Areas 44, 45, and 47 and subtracting out the medial portions. Left and right ROIs for all regions were constructed and assessed for activation and connectivity separately.
3. Results
3.1 Behavioral results
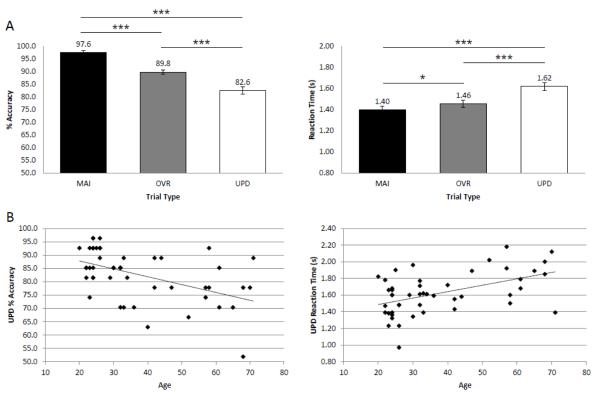
An ANOVA demonstrated a significant main effect of condition on Accuracy (UPD Accuracy ± SD: 82.6 ± 9.6%; MAI Accuracy ± SD: 97.6 ± 4.6%; OVR Accuracy ± SD: 89.8 ± 6.0%; F(2,92)=51.76, p<0.001). Post-hoc paired-samples t-tests revealed significantly decreased UPD accuracy compared to both OVR (p<0.001) and MAI (p<0.001), and significantly decreased OVR accuracy compared to MAI (p<0.001). There was also a significant main effect of condition on reaction time (UPD RT ± SD: 1.62 ± 0.25; MAI RT ± SD: 1.40 ± 0.26; OVR RT ± SD: 1.46 ± 0.23; F(2,92)=35.46, p<0.001). Post-hoc paired-samples t-tests revealed significantly increased UPD reaction time compared to both OVR (p<0.001) and MAI (p<0.001), and significantly increased OVR reaction time compared to MAI (p<0.05) (Figure 2A).
Figure 2. Behavioral results.
One-way ANOVAs revealed main effects of condition on task accuracy and reaction time; post-hoc paired-samples t-tests showed that UPD was the most difficult condition (p<0.05*, p<0.01**, p<0.001***) (A). Aging correlated with lower % Accuracy (r=−0.494, z=−0.541, p<0.001) and longer Reaction Times (r=0.495, z=0.543, p<0.001) during UPD (B).
Increasing age significantly correlated with accuracy only during the Updating condition, in which increasing age was associated with lower accuracy (r=−0.494, z=−0.541, p<0.001) and longer reaction times (r=0.495, z=0.543, p<0.001) (Figure 2B). Aging did not correlate with performance during MAI (accuracy: r=0.120, z=0.1206; reaction time: r=0.210, z=0.213) or OVR (accuracy: r=0.116, z=0.116; reaction time: r=0.208, z=0.211) conditions. Further, the Fisher’s r to z transformation demonstrated an Age x Condition interaction for Accuracy between UPD and MAI (p<0.01) and UPD and OVR (p<0.01), indicating that there was a stronger Age x Accuracy correlation during UPD compared to the other two conditions. There was a similar trend towards Age x Condition interactions for RT between UPD and MAI (p=0.065) and UPD and OVR (p=0.058).
3.2 Fmri Activation Results
3.2.1 Effect of task condition
The main effect of UPD>baseline revealed activation bilaterally in DLPFC, VLPFC, anterior cingulate cortex (ACC), premotor, parietal, and occipital cortices, basal ganglia (including caudate, putamen, globus pallidus, and subthalamic nucleus), SN/VTA, thalamus, and cerebellum. The main effect of OVR>baseline also revealed activation bilaterally in DLPFC, VLPFC, ACC, premotor, parietal, and occipital cortices, basal ganglia (caudate, putamen, globus pallidus, and subthalamic nucleus) SN/VTA, thalamus, and cerebellum. The main effect of MAI>baseline included the bilateral DLPFC, VLPFC, ACC, premotor, parietal, and occipital cortices, putamen, thalamus, and cerebellum.
The group analysis of UPD>OVR showed significant activation bilaterally in DLPFC, VLPFC, ACC, premotor, parietal, and occipital cortices, basal ganglia (including caudate, putamen, globus pallidus, and the subthalamic nucleus), SN/VTA, thalamus, and cerebellum.
3.2.2 Effects of age
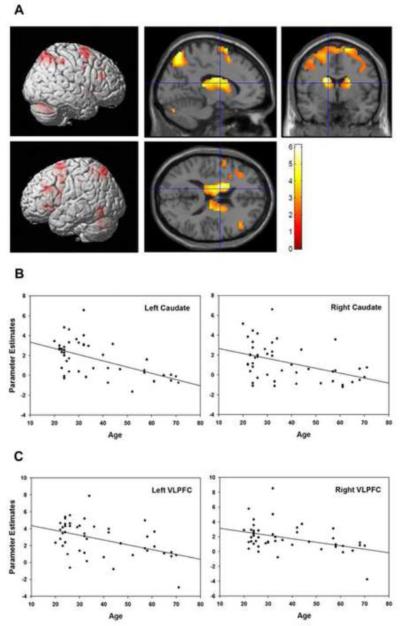
Using age as a regressor with the UPD>OVR contrast, masked for task-related activation (as described in the statistical methods section), revealed that older age was associated with lower activation in the bilateral basal ganglia (caudate, putamen, and globus pallidus),DLPFC, VLPFC, ACC, premotor, and parietal cortices and the left SN/VTA (Figure 3, Table 1). No areas showed greater activation with increasing age in these task-relevant regions. Two-sample analyses comparing younger and older subjects’ UPD>OVR contrast demonstrated similar findings (see supplementary materials for details). Supplemental analyses of age-related effects in UPD, OVR, and MAI compared to baseline demonstrated similar age-related activation reductions during UPD, but not OVR or MAI. A significant increase in activation with age was observed during MAI in the right putamen.
Figure 3. Effect of age on neural activity underlying updating (UPD>OVR contrast).
(A) Older age was associated with reduced activation (p<0.05, whole-brain FDR-corrected, masked for main effect of task: p<0.05, uncorrected). (B) Scatterplots show relationship between age (in years) and parameter estimates of the BOLD response (measured in arbitrary units) in the left caudate (r=−0.56, p<.0001), right caudate (r= −0.43, p<0.003), left VLPFC (r= −0.45, p=0.001), and right VLPFC (r= −0.41, p=0.004).
Table 1.
Older age associated with reduced neural activation underlying updating (UPD>OVR contrast)
| Region | # of voxels |
Peak Z score |
p value |
Correct ed p value |
x | y | z |
|---|---|---|---|---|---|---|---|
| Anterior Cingulate - R | 76 | 4.91 | 0.000 | 0.000 | 9 | 12 | 51 |
| Caudate - L | 152 | 4.95 | 0.000 | 0.000 | −18 | 3 | 18 |
| Caudate - R | 102 | 3.64 | 0.000 | 0.001 | 18 | −3 | 21 |
| DLPFC - L | 88 | 3.61 | 0.000 | 0.010 | −42 | 27 | 24 |
| DLPFC - L | 62 | 3.22 | 0.001 | 0.010 | −45 | 3 | 27 |
| DLPFC - R | 60 | 3.45 | 0.000 | 0.010 | 48 | 30 | 21 |
| Globus Pallidus - L | 9 | 3.43 | 0.000 | 0.007 | −15 | 3 | 9 |
| Globus Pallidus - R | 12 | 3.03 | 0.001 | 0.007 | 18 | −6 | 9 |
| Lateral Parietal Cortex - L | 42 | 2.72 | 0.003 | 0.007 | −33 | −57 | 51 |
| Lateral Parietal Cortex - R | 174 | 4.98 | 0.000 | 0.000 | 33 | −57 | 48 |
| Medial Parietal Cortex - bilat | 795 | 5.18 | 0.000 | 0.000 | 30 | −57 | 48 |
| Premotor Cortex - bilat | 879 | 4.91 | 0.000 | 0.000 | 9 | 12 | 51 |
| Putamen - L | 139 | 4.71 | 0.000 | 0.000 | −18 | 3 | 15 |
| Putamen - R | 80 | 3.46 | 0.000 | 0.003 | 21 | −3 | 18 |
| Substantia Nigra - L | 6 | 2.42 | 0.008 | 0.014 | −6 | −21 | −12 |
| Thalamus - bilat | 305 | 3.96 | 0.000 | 0.002 | −15 | −9 | 18 |
| VLPFC - L | 78 | 3.65 | 0.000 | 0.007 | −36 | 27 | 3 |
| VLPFC - L | 5 | 3.10 | 0.001 | 0.008 | −45 | 27 | 24 |
| VLPFC - L | 31 | 3.00 | 0.001 | 0.008 | −51 | 6 | 21 |
| VLPFC - R | 12 | 3.34 | 0.000 | 0.007 | 48 | 27 | 24 |
| VLPFC - R | 37 | 2.77 | 0.003 | 0.010 | 36 | 15 | −9 |
p<0.05, whole-brain FDR-corrected; significant clusters isolated using regional masks derived from WFU PICKATLAS tool
3.2.3 Effects of performance
Regression analyses of percent change in task accuracy between UPD and OVR revealed that activation in the bilateral caudate predicted stable or improved performance in UPD relative to OVR (Figure 4A, Table 2). For the reaction time analysis, activation in the bilateral VLPFC correlated with faster UPD relative to OVR reaction times (Figure 4B, Table 3). As in the aging analyses, these performance analyses were limited to regions showing task-related activation.
Figure 4. Relationships between behavioral performance and neural activation during Updating (UPD>OVR contrast).
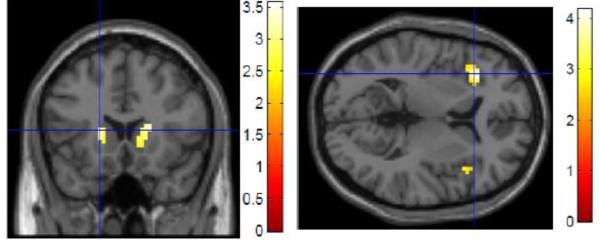
Activation in the bilateral caudate was associated with similar or improved accuracy in UPD compared to OVR (A); activation in the bilateral VLPFC was associated with similar or improved reaction time in UPD compared to OVR (B) (p<0.05 ROI/small volume FDR-corrected, masked for main effect of task: p<0.05, uncorrected)
Table 2.
Activation in the bilateral caudate (UPD>OVR contrast) associated with similar or improved accuracy in UPD compared to OVR
| Region | # of voxels | Peak Z score |
p value | Corrected p value |
x | y | z |
|---|---|---|---|---|---|---|---|
| Caudate - L | 98 | 3.32 | 0.000 | 0.028 | −18 | 18 | 9 |
| Caudate - R | 35 | 3.08 | 0.001 | 0.028 | 21 | 18 | 12 |
ROI analysis using WFU PICKATLAS tool
Table 3.
Activation in the bilateral VLPFC (UPD>OVR contrast) associated with similar or improved reaction time in UPD compared to OVR
| Region | # of voxels | Peak Z score |
p value | Corrected p value |
x | y | z |
|---|---|---|---|---|---|---|---|
| VLPFC - L | 48 | 3.80 | 0.000 | 0.020 | −36 | 24 | 6 |
| VLPFC - R | 6 | 3.04 | 0.001 | 0.042 | 42 | 18 | 9 |
ROI analysis using WFU PICKATLAS tool
3.3 fMRI connectivity results
3.3.1 Effect of task condition
During UPD compared to baseline, the left VLPFC showed significant functional coupling with the bilateral VLPFC, DLPFC, ACC, superior temporal gyrus, inferior posterior parietal cortex, medial PFC, basal ganglia, and thalamus, with left PFC connectivity predominating. The right VLPFC showed a similar bilateral pattern of coupling, with the right PFC predominating. The left caudate showed significant functional “connectivity” with the bilateral basal ganglia (including caudate, putamen, and midbrain SN/VTA region), orbito-frontal cortex (OFC), VLPFC, DLPFC, superior parietal cortex, lateral occipital cortex, and thalamus. The right caudate showed a similar pattern of connectivity.
3.3.2 Effects of Age and Performance
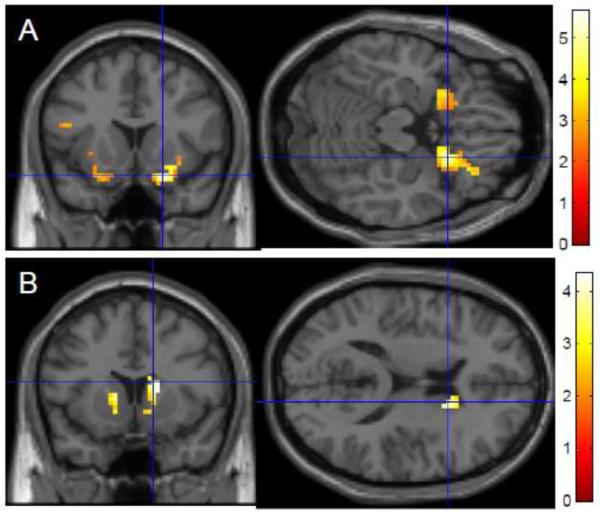
The left caudate seed showed reduced connectivity with the left VLPFC and the rest of the caudate with increasing age, and the right caudate seed additionally showed reduced connectivity with the bilateral VLPFC with increasing age. The right VLPFC seed showed significant reduced connectivity with advanced age in the bilateral caudate and the rest of the right VLPFC, while the left VLPFC achieved trend-level significant age-related connectivity reductions in the caudate bilaterally (Figure 5, Table 4). Performance during UPD did not significantly correlate with functional connectivity.
Figure 5. Age-related reductions in functional connectivity during Updating: selected representative maps of seed-to-voxel connectivity changes with age (ROI-masked).
Functional connectivity with the left caudate seed region significantly decreased with age in the VLPFC bilaterally (A). Functional connectivity with the right VLPFC seed region significantly decreased with age in the caudate bilaterally (B).
Table 4.
Older age associated with reduced functional connectivity between the left and right caudate and the left and right VLPFC during Updating (UPD beta series analysis)
| Seed | Region | # of voxels |
Peak Z score |
p value | Corrected p value |
x | y | z |
|---|---|---|---|---|---|---|---|---|
| Caudate - L | VLPFC - L | 44 | 3.36 | 0.000 | 0.007 | −24 | 12 | −18 |
| VLPFC - R | 115 | 4.87 | 0.000 | 0.000 | 21 | 15 | −18 | |
| Caudate - L | 64 | 3.84 | 0.000 | 0.034 | −12 | 18 | 3 | |
| Caudate - R | 57 | 3.49 | 0.000 | 0.034 | 15 | 12 | 12 | |
| Caudate - R | VLPFC - L | 16 | 2.83 | 0.002 | 0.051 | −21 | 9 | −18 |
| VLPFC - R | 45 | 4.08 | 0.000 | 0.005 | 21 | 15 | −18 | |
| VLPFC - L | Caudate - L | 16 | 2.77 | 0.003 | 0.065 | −12 | 9 | 6 |
| Caudate - R | 28 | 3.31 | 0.000 | 0.059 | 18 | 9 | 12 | |
| VLPFC - R | Caudate - L | 31 | 3.76 | 0.000 | 0.004 | −12 | 12 | 3 |
| Caudate - R | 49 | 4.33 | 0.000 | 0.004 | 15 | 12 | 15 | |
| VLPFC - R | 45 | 4.17 | 0.000 | 0.012 | 21 | 21 | −21 |
ROI analyses using WFU PICKATLAS tool
4. Discussion
These results indicate that selective WM updating engages the meso-cortico-striatal system to a greater extent than nonspecific overwriting, and normal aging is associated with both poorer task performance and reduced activation and functional coupling within this network. Task performance was also correlated with activation in striatal and prefrontal cortical regions, further demonstrating the importance of this system for WM updating success. With aging, then, comes a reduced ability to coherently activate the most efficient neurocognitive system subserving an executive subcomponent of WM.
Our finding of activation during WM updating in the meso-cortico-striatal network implicated in executive cognitive control is consistent with computational models of information gating by the basal ganglia to selectively allow access to online WM representations maintained by prefrontal and parietal cortices (Braver, et al. 2002; Frank, et al. 2001; Frank 2008; Hazy, et al. 2006). As predicted, this study demonstrates that the selective updating of WM relies more heavily on this system than nonspecific overwriting. Regions proposed by computational models to play important roles in executive cognitive control, including midbrain dopamine centers, the dorsal striatum, and the lateral prefrontal cortex, were activated to a greater degree during UPD compared to OVR.
Behavioral evidence for age-related cognitive decline was observed only during the more cognitively demanding UPD condition, when older age correlated with reduced accuracy and longer reaction times. Age-related under-recruitment of the meso-cortico-striatal system was also present only during UPD; this was observed in the UPD>OVR contrast as well as the UPD>baseline contrast.
Within the meso-cortico-striatal network, aging is crucially associated with reductions in UPD>OVR activation in the caudate and VLPFC, regions found to be important for preserving updating performance. Increasing age was not only associated with weaker recruitment but also with reduced functional coupling between these task-relevant regions.
Prefrontal-striatal loops supporting cognition (i.e., connecting the DLPFC and VLPFC with the caudate nucleus) have been anatomically identified in humans (Leh, et al., 2007), and the results of the present study suggest that caudate-VLPFC circuitry in particular may play an important role in WM updating. Converging evidence has previously suggested that the inferior frontal cortex, including VLPFC, is critical for inhibitory control (Aron, et al., 2007,Aron, et al., 2004). This refers to one’s ability to control, or “stop”, motor, cognitive, and emotional processes. Inhibitory control may be important for successful WM updating, as it is necessary to purposefully disregard (or inhibit) previously stored, currently irrelevant information in favor of new incoming information during this process. A network including the Caudate and VLPFC may thus play an important role in a more general executive control mechanism proposed to account for a variety of seemingly distinct cognitive processes such as inhibition, set-shifting, and the executive sub-functions of WM (Braver and Barch, 2002).
We find that behavioral deficits in WM updating are accompanied by reduced utilization of the most efficient (as seen in younger subjects) neurocognitive strategy. The dedifferentiation hypothesis of cognitive aging proposes that brain regions showing specialized responses to specific cognitive tasks become less specialized with increasing age (Goh, 2011). The brain thus responds in a more general manner to all tasks, with decreased activation and functional coupling in task-relevant regions and increased activation in less specialized regions. This may alternatively be viewed as an adaptive compensatory mechanism if these activation and/or connectivity increases are associated with improved or preserved performance ((Cabeza, et al., 2002, Park and Reuter-Lorenz, 2011, Peelle, et al., 2010). In this study, there was an age-related decline of the task-relevant meso-cortico-striatal system with no evidence of increased activation outside this canonical network. Our results, therefore, do not explicitly support the dedifferentiation or compensation hypotheses.
It is not surprising that we see no evidence of compensation since our aging subjects perform worse than younger subjects. Future analyses may compare high- and low-performing older adults or assess age-related effects in a performance-matched sample of younger and older subjects (though such a sample would be difficult to obtain). In the present study, our analyses were limited to task-relevant, age-related changes (main effect-masked), but age-related changes in task-induced deactivations may also affect WM performance (Sambataro, et al., 2010), and future studies using this task may explore this subject.
Given the known neuromodulatory role that dopamine plays within the meso-cortico-striatal system and its proposed importance for executive functions that decline with age (Braver and Barch., 2002), it is possible that age-related changes to the dopamine system may underlie the observed BOLD signal changes. In addition to influencing neural activation, it is also possible that age-related changes to the dopaminergic system may affect the hemodynamic response in the aging brain more generally via neurovascular effects (Choi, et al., 2006), which cannot be assessed here. However, recent studies probing both WM-related activation and dopamine system function support the former idea (Braskie, et al., 2008, Klostermann, et al., 2012).
In one such combined PET and fMRI study, older subjects showed reduced load-dependent modulation of activation in frontal and parietal cortices during a WM task and reduced D1 receptor binding potential in the caudate (Backman, et al., 2011). Controlling for D1 receptor binding potential reductions normalized these fMRI findings, suggesting that age-related alterations in the dopaminergic system may contribute to age-related under-recruitment of relevant brain regions during WM (or vice-versa). A causal role for dopamine depletion in age-related cognitive decline and BOLD signal changes has also been suggested by a recent study in which pharmacological depletion of dopamine in young subjects (via D1 receptor antagonism) affected performance and load-dependent modulation of spatial WM activation in fronto-parietal regions in a way that modeled that of a normal aging population (Fischer, et al.).
In contrast to receptor binding potential, dopamine synthesis capacity is often found to increase with normal aging and may be viewed as a compensatory indicator of a system functioning sub-optimally (Klostermann, et al. 2011; Braskie, et al. 2008). In a PET-fMRI study supporting this, Klostermann and colleagues showed that dopamine synthesis capacity negatively correlates with load-dependent frontal-caudate functional connectivity, which itself decreases with age and correlates with better WM performance (Klostermann, et al. 2011). While the present WM updating study is limited by having no direct measure of dopamine system integrity for these subjects, age-related decreases in fronto-striatal connectivity observed here may similarly relate to changes in the dopaminergic system.
In addition to having no measure of dopamine system function, another limitation of this study was that the sample of 47 individuals was skewed towards younger subjects, in part because of strict medical inclusion criteria. In a supplementary two-sample analysis comparing young (<35) and old (>55) subjects, we found similar age-related activation reductions in the UPD>OVR contrast. Instead of using a two-sample approach to study cognition in elderly, we chose to use a regression approach in order to capture changes related to increasing age occurring over a larger range of the adult aging spectrum, as age-related cognitive changes in healthy adults likely begins as early as one’s 20’s and 30’s (Salthouse 2009).
For the purposes of interpreting the UPD>OVR results, it should be noted that UPD trials were more difficult than OVR trials, as demonstrated by the behavioral analyses. While it is possible that this activation profile may relate more generally to the increased cognitive processing demands required during UPD as compared to OVR, we believe that our activation results are consistent with the distinct cognitive processes occurring between these two conditions, namely, the selective and specific WM gating occurring during UPD.
For WM updating to be successful, the central executive needs to learn how and when to selectively and specifically gate information into the WM store, and this learning is thought to rely on dopaminergic reinforcement mechanisms (Gruber, et al. 2006; Hazy, et al. 2006). Updating may therefore rely on dopaminergic modulation to a greater extent than the other WM subcomponent processes tested here. This may account for the preferential engagement of the meso-cortico-striatal system in UPD>OVR and the fact that age-related effects were observed only in UPD and UPD>OVR analyses.
Individual differences in performance may also relate to the neurocognitive strategies employed by subjects. Future studies are needed to investigate whether subjects use more verbal or visuospatial strategies, and how these strategies may interact with brain activity, task performance, and aging, since it has been suggested that visuospatial working memory is affected by aging to a greater degree than verbal working memory (Jenkins, et al., 2000).
All interpretations of UPD>OVR results rely on the logic of cognitive subtraction, which is limited by its assumption that adding a new cognitive component to a task (e.g., selective WM gating) does not change the instantiation of the pre-existing cognitive task to be subtracted (e.g., OVR) (Friston, et al., 1996, Posner, et al., 1988, Smith and Jonides, 1997).
The cerebellum was not one of our a priori regions of interest, but it nevertheless was engaged during both UPD and OVR, to a greater extent in UPD, and older age was associated with reduced activation in the cerebellum. These results suggest that the cerebellum plays a role in WM updating and that it may be a part of the functional network that declines with age. Indeed, cerebellar volume has been shown to shrink dramatically across adulthood, and its age-related volume change is second only to the caudate (Raz, et al., 2005), and cognitive ability in older adults has been associated with cerebellar volume (MacLullich, et al., 2004). While the cerebellum has traditionally been studied as a brain area responsible for motor behavior and coordination, advancements in the past several decades have led to the view that this region participates in a wide array of nonmotor functions including cognition, which may be mediated by its anatomical connectivity with frontal cortical regions (Ben-Yehudah, et al., 2007,Hoppenbrouwers, et al., 2008,Strick, et al., 2009). More specifically, it has been suggested that the cerebellum contributes to the articulatory rehearsal component of WM via error-driven adjustments and internal timing (Ben-Yehudah, et al., 2007),. These processes may be important for the Updating task.
In summary, the meso-cortico-striatal system is activated during WM updating, a process thought to involve executive cognitive control mediated by dopamine-mediated striatal gating and stabilization of working memory representations. Given the known relationship between aging and dopamine system decline, our findings of age-related under-recruitment and reduced functional coupling within this task-relevant system add to the literature on the neurophysiology both of the central executive and of age-related cognitive decline. Future studies using dopamine system genetic variants and pharmacological manipulations may further clarify the role of dopamine in these age-related changes.
Supplementary Material
Highlights.
We explore age-related modulation of neural circuits during working memory updating
We show age-related decline in activation of meso-cortico-striatal regions
We show age-related decline in functional coupling of meso-cortico-striatal regions
We show that these changes occur specifically during working memory updating
Acknowledgements
We would like to thank Martin Safrin and Kristina Thurin for their assistance with fMRI data collection and Bradley Zoltick for his help with performance data parsing. This work was supported by the National Institute of Mental Health Intramural Research Program, and the study protocol was approved by the Intramural Review Board of the National Institute of Mental Health.
Appendix A: Supplementary two-sample analysis of age-related activation in UPD>OVR
Subjects
A subset of subjects included in this study was selected for a two-sample analysis comparing activation in younger and older subjects in the UPD>OVR contrast in order to supplement our main age-related regression analyses. Groups were comprised of 12 younger (<35 years; mean age: 26.5 years; range: 22 to 34; 9 males) and 11 older (>55 years; mean age: 63.1 years; range: 57 to 71; 4 males). Groups were matched for education (younger mean: 17.7 years; older mean: 18.2 years; p=0.48), IQ (younger mean: 116.7; older mean: 116.7; p=0.98), and handedness (younger mean: 80.8; older mean: 82.7; p=0.90). Gender distribution did not differ significantly (p=0.06 by chi-square), but there were more males in the younger group and more females in the older group.
Behavioral data analysis
Accuracy during UPD and the percent difference in accuracy between UPD and OVR were compared between groups using a two-sample t-test with equal variances.
Functional imaging analysis
Additional second-level analyses were performed using a two-sample t-test comparing younger and older subject groups’ UPD>OVR contrasts. This analysis was masked by a combined map obtained by summing the second-level main effect maps (UPD>OVR contrasts, set to threshold of p<0.05, uncorrected) of the two groups in order to limit these analyses to task-related activations. A second supplementary analysis was similarly performed with scaled UPD-OVR accuracy entered as a covariate for all subjects. Statistical thresholds for these analyses were again set to p<0.05, whole-brain FDR corrected within the main-effect masked regions.
Behavioral results
Older subjects were less accurate during UPD and had lower scaled UPD-OVR accuracy values, though these comparisons did not explicitly reach statistical significance (UPD Accuracy: younger mean 84.9%; older mean 76.8%; p= 0.053; UPD-OVR Scaled Accuracy: younger mean −7.9%; older mean −17.6%; p=0.089).
fMRI activation results
Older subjects demonstrated reduced activation compared to younger subjects in the UPD>OVR contrast in both supplementary 2-sample analyses (Supplementary Figures 1-2, Supplementary Tables 1-2). There were no regions showing increased activation in older compared to younger subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: None of the authors have actual or potential conflicts of interest to disclose.
6. References
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–4. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157(4):635–7. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum. 2007;6(3):193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26(7):809–17. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Clarys D, Bugaiska A, Tapia G, Baudouin A. Ageing, remembering, and executive function. Memory. 2009;17(2):158–68. doi: 10.1080/09658210802188301. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59(10):898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery L, Heaven TJ, Paxton JL, Braver TS. Age-related changes in neural activity during performance matched working memory manipulation. Neuroimage. 2008;42(4):1577–86. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM Axis I Disorders (SCID-I, Research version) Biometric Research Department; New York: 1996. [Google Scholar]
- Fischer H, Nyberg L, Karlsson S, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Backman L. Simulating neurocognitive aging: effects of a dopaminergic antagonist on brain activity during working memory. Biol Psychiatry. 67(6):575–80. doi: 10.1016/j.biopsych.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26(7):874–90. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–60. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci. 2006;20(2):153–66. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139(1):105–18. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Schutter DJ, Fitzgerald PB, Chen R, Daskalakis ZJ. The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: a review. Brain Res Rev. 2008;59(1):185–200. doi: 10.1016/j.brainresrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419(2):113–8. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2(2):234–44. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392(1-2):32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58(4):630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci U S A. 2009;106(52):22552–7. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Dahlin E, Stigsdotter Neely A, Backman L. Neural correlates of variable working memory load across adult age and skill: dissociative patterns within the fronto-parietal network. Scand J Psychol. 2009;50(1):41–6. doi: 10.1111/j.1467-9450.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18(2):283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128(Pt 9):1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43(1):65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Reed JD, Murty VP, Das S, Tan HY, Callicott JH, Weinberger DR, Mattay VS. Catechol-O-methyltransferase valine(158)methionine polymorphism modulates brain networks underlying working memory across adulthood. Biol Psychiatry. 2009;66(6):540–8. doi: 10.1016/j.biopsych.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27(49):13393–401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.