Figure 1.
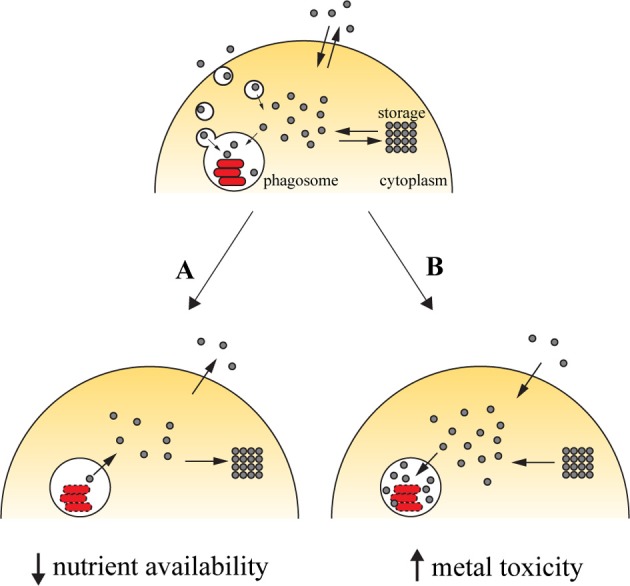
Transition metals in immunity against intracellular pathogens. Upon entry into the host cell, the pathogen is able to retrieve metals from its surroundings to incorporate them in mettaloproteins strictly required for its survival. The pathogen uses dedicated transporters and specialized proteins [e.g., siderophores (Hider and Kong, 2010), high-affinity iron chelating compounds] to acquire metals from the cytoplasmic pools or by hijacking the cell pathways of metal acquisition (for example, Mycobacterium and Leishmania have access to transferrin-bound iron in the endocytic pathway). One of the countermeasures employed by the host cell to reduce the proliferation of the pathogen is metal deprivation (A). This is achieved by pumping out the metal from the phagosome, mediated by metal transporters such as SLC11A1, which is recruited to the phagosomal membrane, where it transports iron and manganese out of this compartment. The metal can then be diverted into storage [e.g., the iron-storage ferritin is induced during infection with Salmonella (Nairz et al., 2007) and Mycobacterium (Silva-Gomes et al., 2013b)] or exported [e.g., ferroportin, an iron exporter that localizes to the cellular membrane, is induced in macrophages infected with Mycobacterium (Van Zandt et al., 2008) and Salmonella (Nairz et al., 2007)]. On the other hand, the host cell can explore the toxicity of transition metal and direct them to the microbial invader (B). During infection, metal transporters such as copper transport 1 (CTR1) (White et al., 2009) are induced, and transport the metal from the extracellular environment. Metals are also mobilized from intracellular storage [e.g., macrophages mobilize zinc from intracellular stores when infected with M. tuberculosis or E. coli (Botella et al., 2011)], which will then accumulate in the phagosome by the action of metal transporters, such as the copper transporter ATP7A (White et al., 2009).
