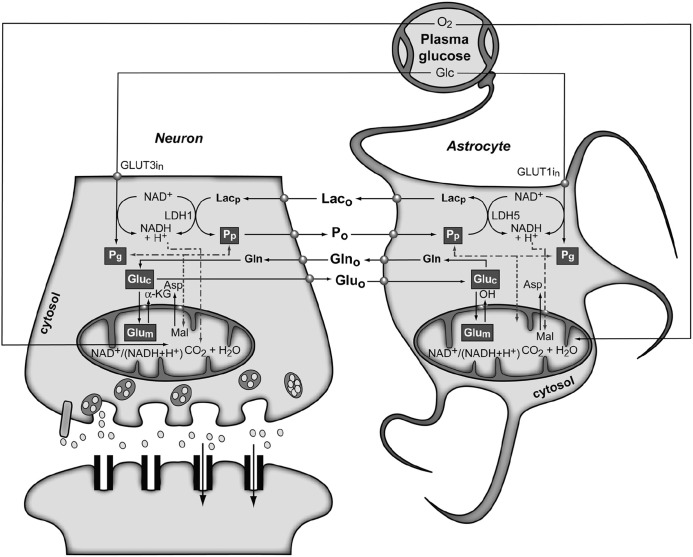
FIGURE 4.
The subcellular compartmentation of pyruvate and glutamate and the redox switch/redox coupling hypothesis. Two pools ofpyruvate exist in neurons and astrocytes derived from extracellular monocarboxylates (Pp) or glucose (Pg). A lactate/pyruvate redox shuttle is able to transfer continuously lactate from astrocytes to neurons, taking advantage of the kinetics of plasma membrane transporters and lactate dehydrogenase isoenzymes. High cytosolic lactate concentration inhibits neuronal glycolysis at the glyceraldehyde-3-phosphate dehydrogenase step by competition with cytosolic NAD+, favoring the oxidation of extracellular Lac. Neuronal pyruvate is transferred back to the astrocyte to close the transcellular exchange of reducing equivalents. Two α-ketoglutarate/glutamate pools exist in neurons and astrocytes, associated probably to cytosolic and mitochondrial compartments. Exchange of α-ketoglutarate/glutamate between mitochondria and cytosol appears to be slow in the H3 glutamate hydrogen exchange timescale and dependent of the cytosolic and mitochondrial NAD(P)+/NAD(P)H ratios, as determined by the malate–aspartate shuttle. Both glycolysis and oxidative astrocytic metabolism contribute the energy for glutamine production in the astrocytes, indicating that this coupling involves both transcellular and intracellular redox coupling mechanisms that allow the simultaneous operation of glycolysis and oxidation in astrocytes. Asp, aspartate; Glc, glucose; Gln, glutamine; Glu, glutamate; GLUT1 and GLUT3, glutamate transporters 1 and 3; α-KG, α-ketoglutarate; Lac, lactate; LDH1 and LDH5, lactate dehydrogenase 1 and 5; Mal, malate. Reproduced with permission from Rodrigues etal. (2012).