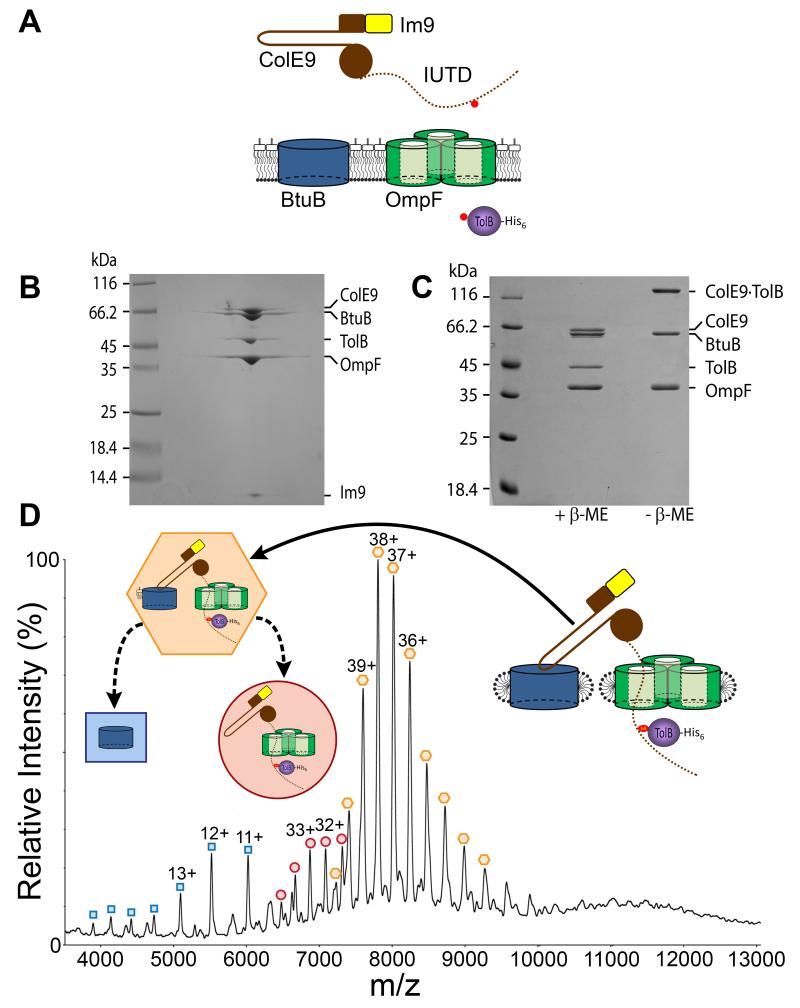
Figure 1. Isolation and characterization of the ColE9 translocon from the OM of Escherichia coli.
(A) Strategy for the capture and isolation of the ColE9 translocon. Nuclease colicins such as ColE9 (brown) contain a hairpin receptor-binding (R−) domain, an N-terminal translocation (T−) domain that includes the intrinsically unstructured translocation domain (IUTD), shown as a dotted line, and a C-terminal cytotoxic DNase domain to which is bound the immunity protein Im9 (yellow). The engineered cysteines for disulfide bond formation between the TolB binding epitope of ColE9 and histidine-tagged TolB (purple) are shown as red circles. (B) BN-PAGE of the isolated translocon showing the complex contains the ColE9-Im9 complex, BtuB, OmpF and TolB. (C) 12% SDS-PAGE of the translocon ± β-mercaptoethanol (β-Me) confirming the presence of the disulfide between ColE9 and TolB. (D) Native-state ESI-MS spectrum of the ColE9 translocon. Coloured inserts give assignments for species observed in the spectrum, all devoid of detergent: orange hexagon, the intact complex that includes a single molecule of lipopolysaccharide; red circle, translocon from which BtuB and lipopolysaccharide have dissociated in the gas phase; blue square, dissociated BtuB. See Table S2 for masses.