Figure 2. Crystal structures of NS3 complexes.
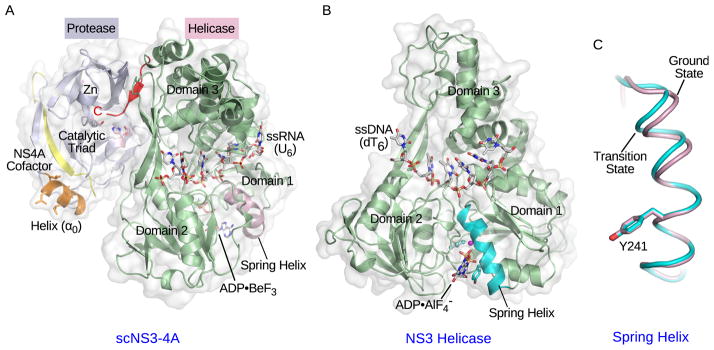
(A) Crystal structure of single-chain NS3-4A (scNS3-4A) in complex with ssRNA (U6) and ADP•BeF3 (PDB: 3O8R). The protease domain, helicase domain, NS4A cofactor peptide, and amphipathic α-helix (α0) are colored light blue, pale green, yellow and orange respectively. The catalytic triad residues in the protease domain are highlighted in light pink. The pink sphere represents the zinc ion. The C-terminal peptide bound to the protease catalytic site is highlighted in red. The ssRNA strand and ADP•BeF3 are shown as stick models. The spring helix is highlighted in light pink. (B) Crystal structure of the NS3 helicase domain in complex with ssDNA (dT6) and ADP•AlF4− (PDB: 3KQL). The helicase domain is colored pale green and the spring helix is highlighted in cyan. The ssDNA strand and ADP•AlF4− are shown as stick models. Three helicase subdomains are labeled in (A) and (B). (C) The spring helices from the ground-state (light pink, PDB: 3KQU) and transition-state (cyan, PDB: 3KQL) complexes are aligned through motif Y and the adjacent residues. The structure presentations were generated by PyMOL (www.pymol.org).
