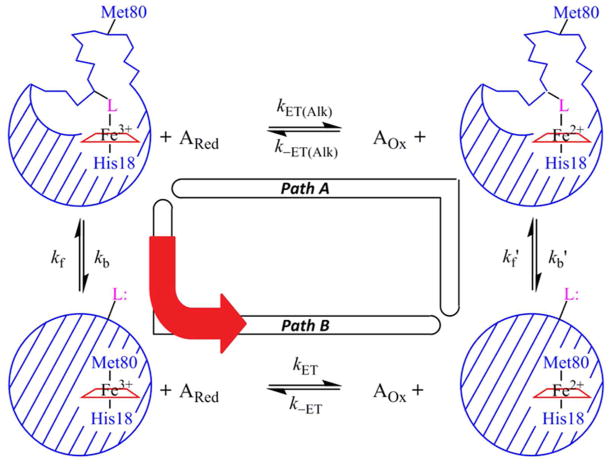
Figure 3.
Kinetic square-scheme for conformationally-gated ET between iso-1-Cytc and small inorganic reagents in the reduced, ARed, and oxidized, AOx, states. Iso-1-Cytc can be in either its native state with Met80 bound to the heme or in an alkaline conformer with an alternate ligand, L, bound to the heme. L is either His79 or Lys73 in the current work. In Path A, the Fe3+-heme alkaline conformer is reduced first to the Fe2+-heme alkaline conformer and then switches the His79 or Lys73 heme ligand for Met80 to form the native conformational state. The reduction of the oxidized alkaline conformer is controlled by the bimolecular rate constants kET(Alk) and k−ET(Alk). The rate constant for converting the reduced alkaline conformer to the reduced native conformer is kb′ and for converting the reduced native conformer to the reduced alkaline conformer is kf′. In Path B, gated ET, the Fe3+-heme alkaline conformer first exchanges its ligand to reach the Fe3+-heme native conformer before it is reduced. The rate constant for converting the oxidized alkaline conformer to the oxidized native conformer is kb and for converting the oxidized native conformer to the oxidized alkaline conformer is kf. (This assignment of kf and kb matches that typically used with the alkaline conformational transition of Cytc.) The reduction of the oxidized native conformer is controlled by the bimolecular rate constants kET and k−ET. This figure is adapted from reference 14.