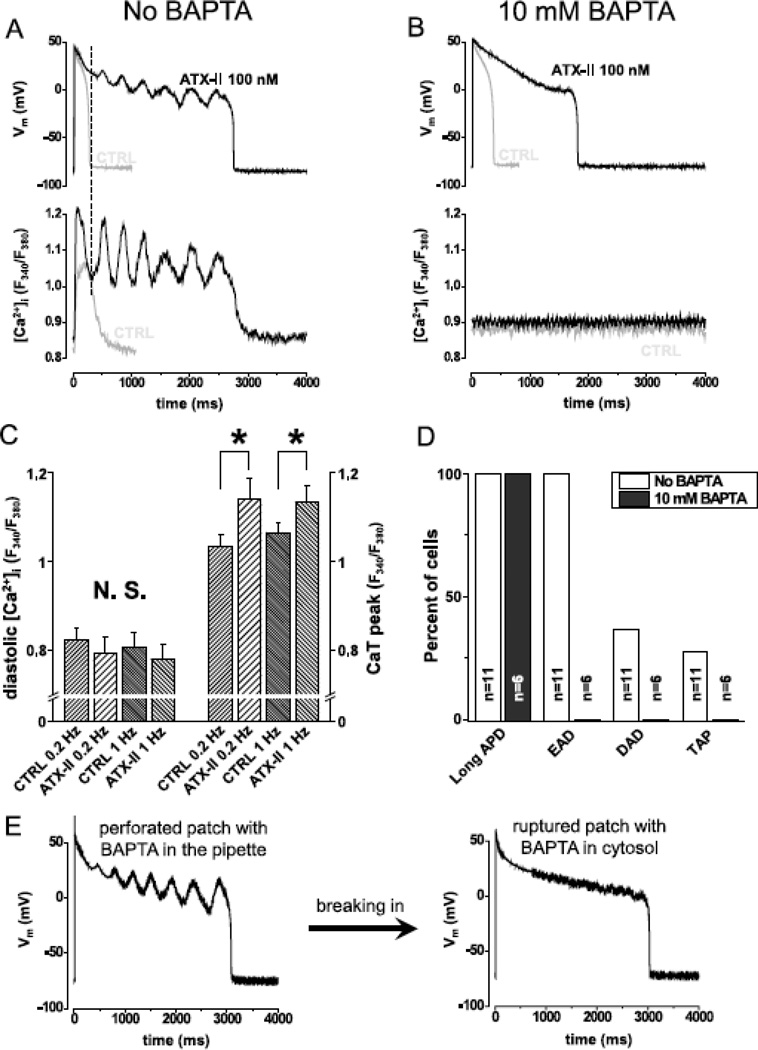
Fig.5. Spontaneous Ca2+ oscillation gives rise to EAD.
Panel A shows simultaneous recording of AP (upper trace) and Ca2+ concentration (lower trace) under sAP-clamp (n=9cells/5animals). Grey and black lines show traces recorded in the absence and the presence of 100 nM ATX-II, respectively. ATX-II lengthened APD but EADs were seen only when Ca2+ was not buffered. Dashed line in panel A shows that the rise in Ca2+ signal precedes the turning point of membrane potential indicating that Ca2+ drives EAD. Panel B shows that when Ca2+ was clamped to 100 nM using BAPTA (n=6cells/3animals), ATX-II lengthened AP but no EAD was observed (grey line control, black line ATX-II). Statistical analysis of the Ca2+ transients (Panel C,) reveal that systolic, but not diastolic Ca2+ concentration was increased by ATX-II at 0.2 Hz (n=9cells/5animals) and 1.0 Hz (n=8cells/5animals) pacing rate. Panel D shows the percent of cells developing various forms of arrhythmogenic APs in the presence of 100 nM ATX-II. When Ca2+ cycling was not buffered (n=11cells/6animals), all cells developed long APD and EADs and some developed DADs and TAPs. When Ca2+ was buffered with BAPTA (n=6cells/3animals), the cells developed long APD, but no EAD, DAD or TAP was seen.