Abstract
Previous studies have reported an increased risk of myocardial infarction (MI) associated with acute increases in PM concentration. Recently, we reported that MI/fine particle (PM2.5) associations may be limited to transmural infarctions. In this study, we retained data on hospital discharges with a primary diagnosis of acute myocardial infarction (using International Classification of Diseases 9th Revision [ICD-9] codes), for those admitted January 1, 2004 to December 31, 2006, who were ≥18 years of age, and were residents of New Jersey at the time of their MI. We excluded MI with a diagnosis of a previous MI and MI coded as a subendocardial infarction, leaving n=1563 transmural infarctions available for analysis. We coupled these health data with PM2.5 species concentrations predicted by the Community Multiscale Air Quality chemical transport model, ambient PM2.5 concentrations, and used the same case-crossover methods to evaluate whether the relative odds of transmural MI associated with increased PM2.5 concentration is modified by the PM2.5 composition/mixture (i.e. mass fractions of sulfate, nitrate, elemental carbon, organic carbon, and ammonium). We found the largest relative odds estimates on the days with the highest tertile of sulfate mass fraction (OR=1.13; 95% CI = 1.00, 1.27), nitrate mass fraction (OR=1.18; 95% CI = 0.98, 1.35), and ammonium mass fraction (OR=1.13; 95% CI = 1.00 1.28), and the lowest tertile of EC mass fraction (OR=1.17; 95% CI = 1.03, 1.34). Air pollution mixtures on these days were enhanced in pollutants formed through atmospheric chemistry (i.e., secondary PM2.5) and depleted in primary pollutants (e.g., EC). When mixtures were laden with secondary PM species (sulfate, nitrate, and/or organics) we observed larger relative odds of myocardial infarction associated with increased PM2.5 concentrations. Further work is needed to confirm these findings and examine which secondary PM2.5 component(s) is/are responsible for an acute MI response.
INTRODUCTION
Previous studies investigating triggering of myocardial infarction (MI) by particulate air pollution (PM) concentrations in the hours and days before MI onset have, in most cases, reported an increased risk of MI associated with increases in PM concentration on the same and previous day.1–9 Recently, we reported that these MI/fine particle (PM2.5) associations may be limited to full wall infarctions (i.e. transmural infarctions), and not subendocardial infarctions (i.e. non-transmural infarctions). Further, this association was independent of increases in nitrogen dioxide, sulfur dioxide, carbon monoxide, and ozone concentrations. 7 Whether the PM2.5 composition/mixture (i.e. relative proportion of PM2.5 mass that is sulfate, nitrate, ammonium, elemental carbon, or organic carbon) modifies this association has not been examined.
National studies have reported regional differences in PM mediated cardiovascular health effects, with larger relative risks of mortality or morbidity observed in the eastern US than in the western US.10–14 These differences may be due to exposure error resulting from regional differences in the efficiency with which ambient PM2.5 penetrates into and persists indoors.15–18 Alternatively, others have argued that differences in effect estimates across regions of the United States occur because of differential effects of PM2.5 species/components. These studies of health effects associated with individual PM components (e.g. sulfates, nitrates, elemental carbon, etc.) have been summarized previously.19 In some cases, secondary species (i.e., formed through atmospheric photochemistry, e.g., sulfate) have been implicated and others have implicated primary species. Studies have reported greater mortality rates on days with elevated primary nickel, vanadium, and elemental carbon,10 primary nickel, vanadium, and secondary sulfate concentrations across 60 US cities,20 primary silicon, aluminum, and arsenic across 25 US cities,12 and primary bromine, chromium, and sodium ion across 27 US cities.21 Others have directly estimated the change in mortality or morbidity associated with specific PM species and reported associations between elemental carbon and cardiovascular (CV) mortality in Phoenix, Arizona,22 elemental carbon, organic carbon (primary plus secondary), iron, and potassium and CVD mortality in California,23 and elemental carbon and CV hospitalizations across 119 US communities.24 Recently, Kim et al (2012) reported that increased EC and OC, but not nitrate and sulfate, were associated with increased ischemic heart disease admissions in Denver, Colorado.25 Although one or more PM component(s) does not clearly stand out as driving these air pollution mediated cardiovascular effects, these studies suggest that mixtures enhanced with specific particle components may be associated with a larger biologic response than others.
Our primary analysis7 took advantage of continuous PM2.5 monitoring at 7 monitoring sites across New Jersey, but limited monitoring of PM2.5 species (i.e. only every 3rd day) did not permit us to examine how acute CV responses are affected by variations in PM2.5 composition. Therefore, we used PM2.5 species concentrations predicted by the Community Multiscale Air Quality chemical transport model,26 ambient PM2.5 mass concentrations measured at 7 continuous monitoring sites across the state, and the same case-crossover design and dataset as in our previous analysis,7 to estimate the relative odds of transmural MI associated with increased PM2.5 concentration on days with varying PM compositions. In this work, we did not estimate the relative odds of transmural MI associated with increases in the concentration of individual components, but rather focused on the whether the relative odds of MI associated with increased PM2.5 concentration was different on days with differing particle composition (i.e., different proportions of PM2.5 mass that were sulfate, nitrate, ammonium, elemental carbon, and organic carbon). We hypothesized that the relative odds of transmural MI associated with increased PM2.5 concentration would be modified by the composition of PM2.5 (i.e. mass fractions of sulfate, nitrate, ammonium, elemental carbon, organic carbon).
METHODS
Study population
The study population and definition of transmural infarction used in this study have been described previously.7 Briefly, we used the Myocardial Infarction Data Acquisition System (MIDAS), a New Jersey statewide database that combines hospital discharge data and death certificate registration data,27 and extracted all records with a primary diagnosis of acute myocardial infarction (International Classification of Diseases 9th Revision [ICD-9] code 410.01, 410.11, 410.21, 410.31, 410.41, 410.51, 410.61, 410.71, 410.81, 410.91), for patients who were admitted between January 1, 2004 and December 31, 2006, were ≥18 years of age, and were residents of NJ at the time of their MI. We excluded those MI with a diagnosis of a previous MI, and those MI coded as a subendocardial infarction (410.7), leaving n=1563 transmural infarctions available for analysis. This study was approved by the University of Medicine and Dentistry of New Jersey Institutional Review Board and the University of Rochester Research Subjects Review Board. MIDAS was also approved by the New Jersey Department of Health and Senior Services Institutional Review Board.
Ambient PM2.5 and weather data
We used the same PM2.5 mass concentrations and weather data for each subject as in the previous analysis of these data.7 In summary, we used ambient hourly PM2.5 mass concentrations, measured with a tapered element oscillating microbalance (TEOM), retrieved from a United States Environmental Protection Agency website,28 for 7 monitoring stations for the study period (January 1, 2004 to December 31, 2006). For each patient, we assigned TEOM PM2.5 measurements from the closest monitor to their residence, with those living greater than 10 km from a PM2.5 monitoring station excluded from all analyses (i.e. the same study subjects as in our previous work7). We assigned hourly temperature and dew point measurements from the weather station (Newark, Caldwell, Somerset, and Trenton airports) closest to each patient’s residence, and then calculated the mean apparent temperature8, 29 in the 24 hours before the MI, as a measure of each patient’s perceived air temperature given the humidity, and used these values in all analyses.
Modeled PM2.5 Species
Ambient PM2.5 species concentrations used in this study (sulfate [SO42−], nitrate [NO3−], ammonium [NH3+], elemental carbon [EC], organic carbon [OC] and remaining other PM2.5 mass) were simulated with CMAQ model Version 4.7. This model used MM5 Version 3.7.4 meteorology (34 vertical layers) and gridded emissions of primary PM2.5 and precursors to secondary PM2.5.26, 30 The National Emissions Inventory (NEI) was the primary basis for emissions. Hour-specific continuous emission monitoring systems data were used for electric generating units. Hour-specific updates to mobile emissions were performed using the MOBILE6 model, and daily estimates of fire emissions based on satellite detection of fires were included. Monthly NH3 emissions from livestock were by inverse modeling.36 The AERO5 aerosol module,31 Carbon-Bond 05 (CB05) chemical mechanism with chlorine chemistry extensions,32 and the ACM2 PBL scheme33, 34 were also used. CMAQ simulations were performed with 36 km continental horizontal grid spacing and 12 km grid resolution for the eastern two-thirds of the U.S. Chemical boundary conditions were obtained from GEOS-Chem.35 Aerosol transport, atmospheric chemistry and secondary PM2.5 formation were simulated to provide hourly CMAQ PM2.5 species concentrations.
CMAQ is known to exhibit seasonal biases in its PM2.5 mass and PM2.5 species outputs.37 To address this, the CMAQ output used in this study was adjusted using a statistical space/time bias-correction model.38 Briefly, EPA’s PM2.5 mass and species data from New Jersey and surrounding states were used to correct the CMAQ bias on spatial scales of 50 km and temporal scales of one month. The model regressed each monitor observation (PM mass or species) on the CMAQ output for the appropriate PM2.5 component, grid cell, and day and used these relationships to adjust the CMAQ concentrations. Biases were allowed to vary in space and time through the use of quadratic splines, and the model was constrained by requiring predicted concentrations to be non-negative and requiring mass closure (sum of species equal total PM2.5 mass). Bias-correction substantially attenuated the seasonal bias trends for all species. For example, after bias correction, the maximum absolute monthly mean bias in the PM2.5 mass concentration (model minus monitor) decreased from 6 μg/m3 to 1 μg/m3. The maximum absolute monthly mean bias for nitrate and organic carbon was reduced from 2.1 μg/m3 to 0.8 μg/m3 and from 3.3 μg/m3 to 1.0 μg/m3, respectively.
Hourly CMAQ concentrations were averaged over each day of the study period. Subjects were assigned these daily surface-level (~0–36 m) PM2.5 mass and PM2.5 species concentrations for the CMAQ grid-cell containing the ambient monitor nearest to their residences. We then used the CMAQ daily concentrations to calculate the daily CMAQ PM2.5 species mass fractions used in the statistical analyses.
Study Design
We used the same time-stratified case-crossover design,39, 40 as in our previous analysis,7 to estimate the risk of a transmural infarction associated with increased TEOM PM2.5 concentrations in the24 hours before emergency department arrival In this design, each patient contributes information as a case during the period immediately before the MI, and as a matched control during times when a MI did not occur. The case-crossover design is analogous to a matched case-control study, but instead of estimating the relative risk of MI contrasting ambient TEOM PM2.5 concentrations between persons (i.e. cases versus controls), we estimate the relative risk of MI contrasting TEOM PM2.5 concentrations during different time periods within the follow-up time of each case of MI. Because case periods and their matched control periods are derived from the same person and a conditional analysis is conducted, non-time varying confounders such as age, co-morbidities, and long term smoking history are controlled by design. However, variables that may be related to both air pollution and the incidence of MI that vary over short time periods (e.g., weather conditions) are possible confounders that must be included in our analytic models. Case periods were defined as the 24 hour period before ER admission for MI, while control periods (3–4 per case depending on the number of days in the calendar month) were matched to the case period by day of the week, time of the day, year, and month. Pollutant concentrations corresponding to these case and control periods are then contrasted in the statistical analyses.
Statistical Analyses - Main Analyses
First, for each day during the study period, we calculated the daily sulfate mass fraction as the CMAQ sulfate concentration (μg/m3) divided by the CMAQ PM2.5 concentration (μg/m3) resulting in a proportion between 0 and 1. We then ranked the sulfate mass fractions for all case and control periods into tertiles (i.e. looking at the proportion of CMAQ PM2.5 mass that is sulfate for each day [sulfate mass fraction with a value from 0 to 1], the HIGH TERTILE equaled the days with the highest third of sulfate mass fractions, the MIDDLE TERTILE equaled the days with the middle third of sulfate mass fractions, and the LOW TERTILE equaled the days with the lowest third of sulfate mass fractions). We repeated this mass fraction calculation and ranking procedure for the nitrate, elemental carbon, organic carbon, and ammonium mass fractions. We then calculated descriptive statistics for these species concentrations and species mass fractions. We also calculated Pearson correlation coefficients for each pair of: TEOM PM2.5; CMAQ sulfate, nitrate, ammonium, elemental carbon, an organic carbon mass fractions; and nitrogen dioxide, carbon monoxide, sulfur dioxide, and ozone concentrations. We repeated this for the summer months (June-August), and winter months (December-February).
Second, we used the same conditional logistic regression model as in our previous analysis7 stratified on each MI, to regress case–control status (i.e., case period = 1, control period = 0) against the mean TEOM PM2.5 concentration in the 24 hr before ED arrival, including a natural spline (3 degrees of freedom) of the mean apparent temperature in the 48 hr before ED arrival in the model. Next, we estimated the risk of a transmural infarction associated with each interquartile range increase (10.8 μg/m3) in TEOM PM2.5 concentration on days with the highest third, middle third, and lowest third of daily sulfate mass fractions. To the same model described above, we added indicator variables for sulfate tertile (MEDIUM_TERTILE + HIGH_TERTILE) and two interaction terms (TEOM PM2.5*MIDDLE_TERTILE _+ TEOM PM2.5*HIGH TERTILE). From this model, we estimated the relative odds of a transmural infarction associated with each 10.8 μg/m3 increase in TEOM PM2.5 concentration when the sulfate mass fraction is in the highest tertile, when it is in the middle tertile, and the lowest tertile. We repeated this analysis for the nitrate, elemental carbon, organic carbon, and ammonium mass fractions.
Third, within each tertile of sulfate mass fraction, we tabulated the mean CMAQ PM2.5 species mass balance (i.e., percent of CMAQ PM2.5 mass that is sulfate, nitrate, elemental carbon, organic carbon, other), and descriptive statistics of gaseous pollutant concentrations, temperature, and dew point. We repeated this for each nitrate, elemental carbon, organic carbon, and ammonium tertile.
Last, we examined the appropriateness of pooling the data from the 7 monitoring sites to estimate the relative odds of a transmural infarction associated with each IQR increase in TEOM PM2.5 in a single statistical model (i.e. our main analysis described above). Alternatively, we could estimate 7 relative odds estimates separately, and then combine them via a meta-analysis technique used previously in a case-crossover study of PM2.5 and mortality in 27 cities.13 Therefore, we added 6 interaction terms to the model described above for the 7 TEOM PM2.5 monitoring sites (e.g. TEOM PM2.5 *Monitoring site #1; TEOM PM2.5*Monitoring site #2, etc.)). We then tested whether the relative odds of transmural MI associated with each IQR increase in TEOM PM2.5 was different for subjects residing near different monitoring locations using a Likelihood Ratio Test. If a Likelihood Ratio Test indicated significant modification of the PM2.5 effect by monitoring site, then we would estimate 7 relative odds estimates separately, combine them using meta-analysis techniques, and compare this estimate to that from our main analysis described above. We used SAS (version 9.1.3; SAS Institute Inc., Cary, NC) and R software (version 2.6.1; R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses.
RESULTS
The characteristics of the patients with a transmural MI included in the study are shown in Table 1. Subjects were predominantly male (63%), white (69%), with 45% 65 years of age and older, and 26% 75 years of age and older. Fifty five percent had hypertension (55%), 27% had diabetes, and 67% had a history of ischemic heart disease (67%).
Table 1.
Frequency and percentage of characteristics of study analysis population (cases matched to PM2.5 monitors at ≤ 10km distance).
| CHARACTERISTIC | Transmural MI (n=1,562) | |
|---|---|---|
| N | % | |
| Age (years) | ||
| 18–44 | 136 | 9 |
| 45–54 | 326 | 20 |
| 55–64 | 404 | 26 |
| 65–74 | 297 | 19 |
| 75–84 | 277 | 18 |
| ≥85 | 122 | 8 |
| Sex | ||
| Male | 979 | 63 |
| Female | 583 | 37 |
| Race | ||
| White | 1,078 | 69 |
| Black | 180 | 12 |
| Other | 304 | 19 |
| Year | ||
| 2004 | 452 | 29 |
| 2005 | 396 | 25 |
| 2006 | 714 | 46 |
| Co-morbidities | ||
| Hypertension | 863 | 55 |
| Diabetes Mellitus | 423 | 27 |
| COPD | 164 | 10 |
| Pneumonia | 60 | 4 |
| Heart Diseases | 1,326 | 85 |
| Ischemic Heart Disease | 1,052 | 67 |
| CHF | 299 | 19 |
| Atrial Fibrillation | 174 | 11 |
| Arrhythmia | 466 | 30 |
| Ventricular Tachycardia | 129 | 8 |
Daily TEOM PM2.5 mass concentrations, bias-adjusted CMAQ PM2.5 and CMAQ species concentrations, and CMAQ species mass fractions (bias-adjusted CMAQ) are summarized in Table 2. We deleted one case from our analysis as the CMAQ PM2.5 and species concentrations were very large due to a large fire in that grid, leaving n=1562 MI for analyses. On average, these five species represented 81% of the total bias-adjusted CMAQ estimated PM2.5 mass concentration. The remainder consisted of minor ions such as sodium and chloride, metal oxides, non-carbon organic mass, and some unspeciated material from primary emission sources (e.g. soil, combustion).41 At the median, sulfate and organic carbon comprised larger proportions of total bias-adjusted CMAQ PM2.5 mass (23% each) than ammonium and nitrate (13%), with elemental carbon being the smallest contributor (9%). Pearson correlation coefficients, provided for each pair of TEOM PM2.5, CMAQ PM2.5 mass fractions, and gaseous pollutant concentrations in Table 3 (entire study period) and Table 4 (summer and winter only), align with expectations based on over 20 years of speciated air quality observations.42 Mass fractions of sulfate, which peak in the summer because of sulfate’s photochemical formation, were moderately, but negatively, correlated with those of nitrate, which is more volatile and enhanced at low temperature (r= − 0.61) and elemental carbon (r= −0.47) which is primary and peaks in the winter. The sulfate mass fraction was positively correlated with ammonium (r=0.51; Table 3). Ammonium was negatively correlated with elemental and organic carbon (r= −0.60 and −0.65 respectively)mass fractions which were weakly, but positively correlated with each other (r=0.35).
Table 2.
Distribution of PM species mass concentrations (CMAQ estimates; seasonally bias adjusted), CMAQ PM2.5 mass; seasonally bias adjusted and TEOM PM2.5 mass concentrations, and PM species mass fractions (CMAQ estimates; Mass Fraction = PM2.5 species conc. / CMAQ PM2.5 conc.) during the study period.
| CMAQ Mass Concentrations (μg/m3) | Mean | Standard Deviation | 5th %tile | 25th %tile | 50th %tile | 75th %tile | 95th %tile |
|---|---|---|---|---|---|---|---|
| Sulfate (SO42−) | 3.1 | 1.9 | 1.4 | 1.9 | 2.5 | 3.6 | 7.6 |
| Ammonium (NH4) | 1.7 | 0.9 | 0.7 | 1.1 | 1.5 | 2.1 | 3.3 |
| Nitrate (NO3) | 1.8 | 1.2 | 0.5 | 0.9 | 1.4 | 2.3 | 4.3 |
| Elemental Carbon (EC) | 1.0 | 0.4 | 0.6 | 0.8 | 1.0 | 1.3 | 1.8 |
| Organic Carbon (OC) | 2.8 | 0.9 | 1.6 | 2.2 | 2.7 | 3.3 | 4.4 |
| CMAQ PM2.5 | 12.5 | 5.2 | 6.1 | 8.5 | 11.3 | 15.2 | 22.3 |
| TEOM PM2.5 | 13.0 | 8.4 | 3.2 | 6.9 | 11.0 | 17.3 | 29.7 |
| CMAQ Mass fractions | |||||||
| Sulfate (SO42−) | 0.25 | 0.07 | 0.15 | 0.19 | 0.23 | 0.29 | 0.39 |
| Ammonium (NH4+) | 0.13 | 0.02 | 0.10 | 0.12 | 0.13 | 0.15 | 0.16 |
| Nitrate (NO3−) | 0.14 | 0.06 | 0.06 | 0.09 | 0.13 | 0.18 | 0.24 |
| Elemental Carbon (EC) | 0.09 | 0.02 | 0.05 | 0.07 | 0.09 | 0.10 | 0.13 |
| Organic Carbon (OC) | 0.24 | 0.05 | 0.16 | 0.20 | 0.23 | 0.27 | 0.34 |
Table 3.
Pearson correlation coefficients for pairs of pollutant concentrations (TEOM PM2.5, NO2, SO2, CO, O3) and CMAQ PM2.5 component mass fractions.
| Pollutant | TEOM PM2.5 | CMAQ Elemental Carbon mass fraction | CMAQ Ammonium mass fraction | CMAQ Nitrate mass fraction | CMAQ Organic Carbon mass fraction | CMAQ Sulfate mass fraction | NO2 | SO2 | CO |
|---|---|---|---|---|---|---|---|---|---|
| TEOM PM2.5 | --- | ||||||||
| CMAQ EC mass fraction | −0.43 | --- | |||||||
| CMAQ Ammonium mass fraction | 0.41 | −0.60 | --- | ||||||
| CMAQ Nitrate mass fraction | −0.01 | −0.12 | 0.15 | --- | |||||
| CMAQ OC mass fraction | −0.49 | 0.35 | −0.65 | −0.42 | --- | ||||
| CMAQ Sulfate mass fraction | 0.33 | −0.47 | 0.51 | −0.61 | −0.22 | --- | |||
| NO2 | 0.46 | 0.00 | 0.18 | 0.39 | −0.43 | −0.17 | --- | ||
| SO2 | 0.44 | −0.14 | 0.11 | 0.43 | −0.43 | −0.23 | 0.57 | --- | |
| CO | 0.35 | 0.09 | 0.07 | 0.23 | −0.32 | −0.07 | 0.64 | 0.49 | --- |
| O3 | 0.19 | −0.32 | 0.22 | −0.43 | 0.11 | 0.45 | −0.45 | −0.33 | −0.39 |
Table 4.
Pearson correlation coefficients for pairs of pollutant concentrations and mass fractions, separately for SUMMER (June, July, August) and WINTER (December, January, February).
| Pollutant | WINTER | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TEOM PM2.5 | CMAQ Elemental Carbon mass fraction | CMAQ Ammonium mass fraction | CMAQ Nitrate mass fraction | CMAQ Organic Carbon mass fraction | CMAQ Sulfate mass fraction | NO2 | SO2 | CO | O3 | ||
| SUMMER | TEOM PM2.5 | --- | −0.20 | 0.19 | 0.23 | −0.50 | −0.08 | 0.71 | 0.66 | 0.62 | −0.54 |
| CMAQ EC mass fraction | −0.47 | --- | −0.36 | −0.49 | 0.21 | 0.10 | 0.05 | −0.22 | 0.09 | −0.07 | |
| CMAQ Ammonium mass fraction | 0.47 | −0.61 | --- | 0.12 | −0.51 | 0.47 | 0.11 | 0.12 | 0.13 | −0.07 | |
| CMAQ Nitrate mass fraction | 0.02 | −0.01 | 0.19 | --- | −0.43 | −0.71 | 0.18 | 0.38 | 0.01 | −0.10 | |
| CMAQ OC mass fraction | −0.57 | 0.41 | −0.68 | −0.19 | --- | 0.09 | −0.39 | −0.50 | −0.28 | 0.42 | |
| CMAQ Sulfate mass fraction | 0.50 | −0.77 | 0.62 | −0.25 | −0.68 | --- | −0.14 | −0.29 | 0.08 | 0.10 | |
| NO2 | 0.33 | 0.01 | 0.20 | 0.31 | −0.32 | 0.06 | --- | 0.62 | 0.71 | −0.63 | |
| SO2 | 0.44 | −0.17 | 0.15 | 0.14 | −0.31 | 0.14 | 0.37 | --- | 0.48 | −0.42 | |
| CO | 0.11 | 0.10 | −0.02 | 0.13 | −0.22 | 0.06 | 0.43 | 0.28 | --- | −0.43 | |
| O3 | 0.59 | −0.40 | 0.34 | −0.06 | −0.22 | 0.24 | −0.13 | 0.19 | −0.30 | --- | |
NO2, SO2 and CO were moderately positively correlated with each other (r>0.5) and weakly but negatively correlated with O3 (r< −0.33). The correlation between O3 and EC mass fraction was weak and negative (r= −0.32). Somewhat stronger associations were observed between O3 and the nitrate (negative; r= −0.43) and sulfate (positive; r=0.45) mass fractions. Similar features were found when correlations were computed by season (Table 4). However, the correlations of sulfate mass fraction with EC (negative; r= −0.77) and ammonium (positive; r=0.62) mass fractions were stronger in the summer-only data. The negative correlation of sulfate with nitrate mass fraction was stronger in the winter (r= −0.71), whereas there was no correlation between EC and sulfate mass fractions in the winter (r=0.10). In the winter, nitrate mass fraction was negatively correlated with EC (r= −0.49), OC (r= −0.43), and sulfate (r= −0.71) mass fractions. The negative correlation between O3 and EC mass fraction was somewhat stronger in the summer-only analysis (r= −0.40) and was not observed in winter. A wintertime positive correlation between O3 and OC mass fraction (r=0.42) is apparent in the seasonally-segregated analysis.
Next, we estimated the relative odds of a transmural infarction associated with each interquartile range increase (10.8 μg/m3) in ambient TEOM PM2.5 concentration in the previous 24 hours within tertiles of sulfate, ammonium, nitrate, elemental carbon, and organic carbon mass fractions on the day of the MI (Table 5). Effect estimates across tertiles were generally similar for sulfate and nitrate, with the largest and only statistically significant (p<0.05) or marginally significant (p<0.10) increased relative odds of a transmural infarction associated with increased TEOM PM2.5 concentration within the highest sulfate and nitrate tertiles. Similarly, the highest and only statistically significant increased relative odds estimate for the ammonium mass fraction was within the highest tertile, with no increased relative odds within the lowest tertile. In contrast, the highest relative odds estimate for the elemental carbon mass fraction was within the lowest tertile. Organic carbon exhibited more complex behavior, which is not surprising considering the vast array of compounds that it contains. The highest relative odds estimate was within the middle tertile of organic carbon mass fraction (Table 5).
Table 5.
Risk (and 95% confidence interval) of a transmural infarction associated with each interquartile range increase in TEOM PM2.5, within each tertile of PM2.5 species mass fraction.
| PM species tertile | Mass fraction | N Total | N Cases | IQR (μg/m3) | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | ||||||||
| Sulfate | Low | 0.094 | 0.206 | 2250 | 494 | 10.8 | 1.08 | 0.92, 1.28 | 0.35 |
| Middle | 0.206 | 0.265 | 2335 | 549 | 10.8 | 1.11 | 0.95, 1.30 | 0.20 | |
| High | 0.266 | 0.531 | 2321 | 519 | 10.8 | 1.13 | 1.00, 1.27 | 0.05 | |
|
| |||||||||
| Ammonium | Low | 0.075 | 0.125 | 2334 | 521 | 10.8 | 1.02 | 0.85, 1.23 | 0.83 |
| Middle | 0.125 | 0.141 | 2297 | 519 | 10.8 | 1.11 | 0.95, 1.29 | 0.18 | |
| High | 0.141 | 0.204 | 2275 | 522 | 10.8 | 1.13 | 1.00, 1.28 | 0.05 | |
|
| |||||||||
| Nitrate | Low | 0.026 | 0.104 | 2427 | 529 | 10.8 | 1.08 | 0.94, 1.23 | 0.28 |
| Middle | 0.104 | 0.167 | 2268 | 541 | 10.8 | 1.11 | 0.97, 1.27 | 0.12 | |
| High | 0.167 | 0.343 | 2211 | 492 | 10.8 | 1.15 | 0.98, 1.35 | 0.08 | |
|
| |||||||||
| EC | Low | 0.027 | 0.078 | 2341 | 535 | 10.8 | 1.17 | 1.03, 1.34 | 0.01 |
| Middle | 0.078 | 0.096 | 2253 | 508 | 10.8 | 1.06 | 0.92, 1.24 | 0.42 | |
| High | 0.096 | 0.192 | 2312 | 519 | 10.8 | 1.07 | 0.90, 1.28 | 0.43 | |
|
| |||||||||
| OC | Low | 0.110 | 0.211 | 2252 | 516 | 10.8 | 1.14 | 1.00, 1.30 | 0.04 |
| Middle | 0.211 | 0.253 | 2258 | 519 | 10.8 | 1.21 | 1.03, 1.42 | 0.02 | |
| High | 0.253 | 0.493 | 2396 | 527 | 10.8 | 0.91 | 0.74, 1.13 | 0.39 | |
NOTE: We regressed case–control status (i.e., case period = 1, control period = 0) against the mean PM2.5 concentration in the 24 hr before ED arrival (calculated from continuous TEOM PM2.5 measurements), including a natural spline (3 degrees of freedom) of the mean apparent temperature in the 48 hr before ED arrival in the model, indicator variables for sulfate tertile (MEDIUM_TERTILE + HIGH_TERTILE) and two interaction terms (PM2.5*MIDDLE_TERTILE _+ PM2.5*HIGH TERTILE). From this model, we estimated the risk of a transmural infarction associated with each 10.8 μg/m3 increase in PM2.5 (TEOM) concentration when the sulfate mass fraction is in the highest tertile, when it is in the middle tertile, and when in the lowest tertile. We repeated this for ammonium, nitrate, EC, and OC.
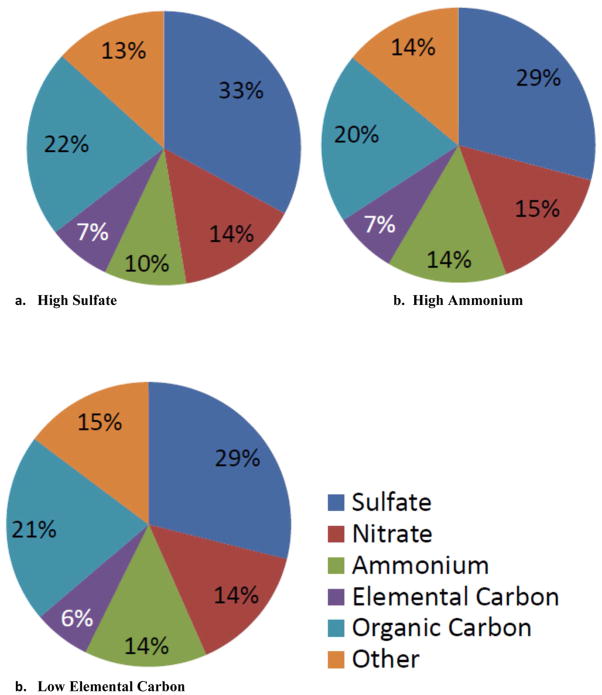
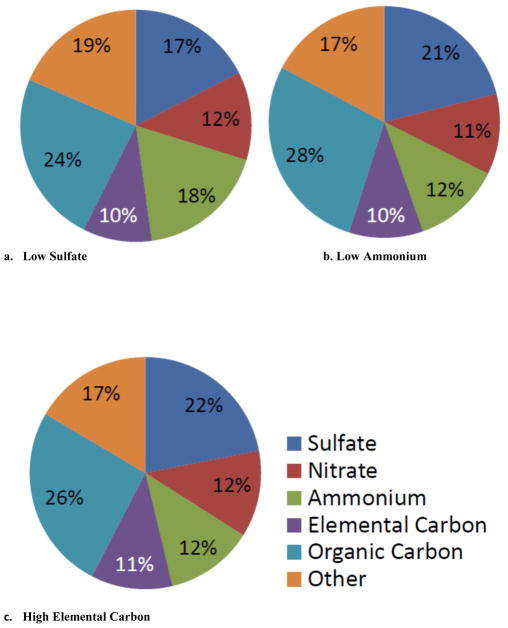
Next, we examined the composition of the pollutant mixture (both PM2.5 composition and gaseous pollutant concentrations) within each tertile. Days in the high sulfate, high ammonium, and low elemental carbon tertiles (Table 6, Figure 1) had very similar compositions. For example, on days in the high sulfate tertile, CMAQ PM2.5 was 33% sulfate, 22% OC, 14% ammonium, 10% nitrate, and 7% EC, on average, and the median temperature and relative humidity were 21.1°C and ~72%, respectively. On these days, median 8 hour maximum NO2, SO2, CO, and O3 concentrations were 24.5 ppb, 5.0 ppb, 0.638 ppm, and 43.6 ppb, respectively (Table 6). Days in the low elemental carbon tertile, had similar CMAQ PM2.5 composition with 29% sulfate, 21% OC, 14% ammonium, 14% nitrate, and 6% EC, on average, and the median temperature and relative humidity were also typical of summertime (18.4°C and ~67% RH). On these days, 8 hour maximum NO2, SO2, CO, and O3 concentrations were also similar to the days with high sulfate (Table 6). In contrast, days in the low sulfate, low ammonium, and high elemental carbon tertiles generally had lower average sulfate mass fractions (17% to 22%), and median 8 hour maximum O3 concentrations (25.7 ppb to 29.3 ppb), but higher average elemental carbon (10% to 11%) and organic carbon mass fractions (24% to 28%); Figure 2 and Table 6). Median 8 hour maximum NO2, SO2, and CO concentrations were similar to the high sulfate, high ammonium, and low elemental carbon tertile days (Table 6). Temperature and median relative humidity were lower and more typical of wintertime.
Table 6.
Median daily gaseous pollutant concentration and weather characteristics, by PM2.5 species tertile
| Figure # | Tertile | NO2 8 hour maximum (ppb) | SO2 8 hour maximum (ppb) | CO 8 hour maximum (ppm) | O3 8 hour maximum (ppb) | Temperature (°C) | Relative Humidity (%) |
|---|---|---|---|---|---|---|---|
| 1 | High Sulfate | 24.5 | 5.0 | 0.638 | 43.6 | 21.1 | 72.1 |
| High Ammonium | 28.6 | 5.9 | 0.688 | 40.3 | 17.4 | 67.8 | |
| Low Elemental Carbon | 26.6 | 6.1 | 0.650 | 43.6 | 18.4 | 67.4 | |
|
| |||||||
| 2 | Low Sulfate | 32.8 | 8.1 | 0.800 | 25.7 | 7.8 | 59.0 |
| Low Ammonium | 27.1 | 5.1 | 0.750 | 29.3 | 14.4 | 63.0 | |
| High Elemental Carbon | 29.6 | 5.3 | 0.838 | 27.9 | 14.1 | 63.0 | |
|
| |||||||
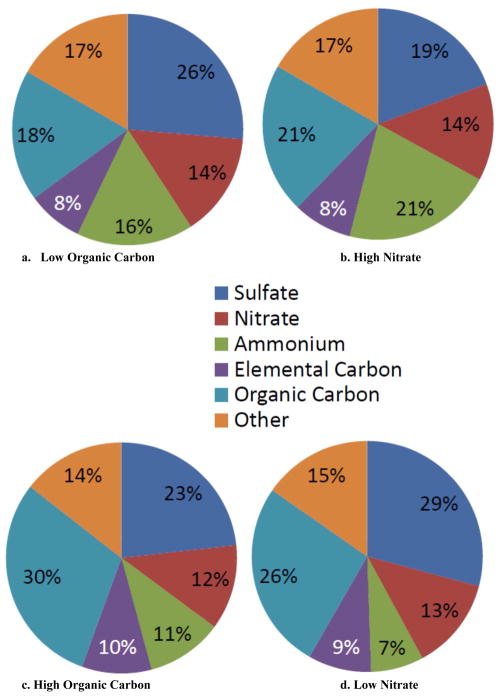
| 3 | High Organic Carbon | 22.4 | 3.8 | 0.600 | 35.0 | 16.4 | 64.2 |
| Low Organic Carbon | 34.4 | 8.6 | 0.838 | 32.6 | 13.6 | 65.9 | |
| High Nitrate | 34.4 | 8.7 | 0.813 | 26.1 | 5.1 | 58.8 | |
| Low Nitrate | 22.3 | 4.4 | 0.613 | 43.5 | 22.3 | 69.1 | |
Figure 1.
Composition of fine particle mass, by PM2.5 species tertile: a. High Sulfate; b. High Ammonium; and c. Low Elemental Carbon
Figure 2.
Composition of fine particle mass, by PM2.5 species tertile: a. Low Sulfate; b. Low Ammonium; and c. High Elemental Carbon
Comparison of high and low nitrate and organic carbon tertiles are presented in Figure 3. In comparison to the high sulfate tertile (Figure 1), the high nitrate tertile was depleted in sulfate and enriched in nitrate, with a substantially colder median temperature (5°C). Note that sulfate (summertime maximum) and nitrate (wintertime maximum) were both associated with ammonium, as these species are frequently present as ammonium salts.42,43
Figure 3.
Composition of fine particle mass, by PM2.5 species tertile: a. Low Organic Carbon; b. High Nitrate; c. High Organic Carbon; and d. Low Nitrate
Days in the low organic carbon tertile had modestly larger contributions of EC and nitrate, a slightly smaller contribution of OC, and a lower median temperature (13.6°C; Figure 3 and Table 3) compared to the high sulfate, high ammonium, and low elemental carbon tertile days (Figure 1). Days in the high organic carbon tertile had larger contributions of elemental and organic carbon, lower contributions of sulfate, nitrate, ammonium, and higher median NO2, CO, and SO2 concentrations then the low organic carbon tertile (Figure 3 and Table 3). The composition of the high organic carbon tertile was different from that of both the high and low sulfate tertiles, presumably either because of differences in sources source regions, or formation chemistry.
Last, we found that the relative odds of a transmural infarction associated with each IQR increase in TEOM PM2.5 concentration in the previous 24 hours was not significantly different across the 7 monitoring sites (all p>0.84). Thus, this meta-analysis approach would not give substantially different results from those described above, supporting our main analysis methodology.
DISCUSSION
Using a combination of daily PM2.5 species mass fractions estimated by the seasonally bias-adjusted CMAQ model, ambient TEOM PM2.5 concentrations from 7 continuous monitoring sites, and MI hospital admissions data across New Jersey from 2004 to 2006, we evaluated whether the relative odds of a transmural infarction associated with each 10.8 μg/m3 increase in TEOM PM2.5 concentration in the previous 24 hours was modified by the PM2.5 composition or mixture (i.e. whether effect of TEOM PM2.5 on MI was different when PM2.5 was composed of a high fraction of sulfate, nitrate, ammonium, EC or OC vs. days when the PM2.5 composition (mixture) contained a low fraction of each of these species). It should be noted that PM2.5 composition simulated on a 12×12 km grid captures the urban mix of primary and secondary PM2.5 species but does not capture the enhanced contribution of primary emissions in close proximity to sources. For example, the contributions to PM2.5 of several carbonaceous species are substantially higher within 100 m of a major roadway (e.g., Polidori et al., 2010).45 The analyses herein are restricted to people who live within 10 km of an urban PM2.5 monitor and include predicted primary and secondary PM2.5. Thus, this paper addresses modification of PM effect estimates by differences in community-level PM2.5 composition, and does not address the enhanced contribution of primary emissions to PM composition in close proximity to primary sources. We found the largest relative odds estimates on the days with the highest tertile of sulfate, nitrate, and ammonium, and the lowest tertile of EC. The air pollution mixtures on the days in these tertiles were all enhanced by PM2.5 pollutant species that are formed through atmospheric chemistry (i.e., secondary PM2.5 formed through gas and/or aqueous photochemistry)and depleted in primary PM2.5 pollutants (in a relative sense, e.g., low EC/PM2.5).
OC exhibited complex behavior, with the highest relative odds estimate occurring for the middle tertile. The complex behavior of OC is not surprising. OC is comprised of thousands of compounds with a wide range of physical and chemical properties. It is both emitted directly (primary)46,47 and also formed in the atmosphere (secondary).48,49 Moreover, EC is a good tracer for primary OC. Sulfate exhibits strong correlations with low volatility oxygenated organic aerosol (a major component of secondary organic aerosol) and oxalate (a tracer for secondary organic aerosol formed through gas followed by aqueous chemistry, known as “aqueous SOA”), probably because all three are formed through atmospheric aqueous chemistry.45,50–53 Additionally, particulate organosulfates are known to form in wet aerosols that contain acidic sulfate.54 Thus a variable portion of OC behaves like nitrate or sulfate, while another portion behaves like EC and total particulate OC is not highly correlated with any other mass fraction (Table 4). Because secondary “aqueous” OC is enriched on days with low EC and high sulfate, this greater acute MI response could be associated with sulfate, nitrate and/or secondary organic aerosol, including SOA formed through aqueous chemistry.
Thus, we found that the relative odds of a transmural MI associated with PM2.5 is greater during times of greater secondary aerosol formation. This is consistent with the hypothesis that mixtures laden with secondary PM species are associated with increased incidence of myocardial infarctions and perhaps with other acute cardiovascular outcomes. Previous studies, done across the United States, have reported increased risk of cardiovascular mortality and morbidity associated with increased sulfate or nitrate concentrations.23, 24, 55,56 Our findings are consistent with these earlier results that have reported greater response to secondary PM species (sulfate, nitrate, and/or organic matter). Note that we did not estimate the relative odds of transmural infarction associated with increases in individual PM2.5 species, and instead assessed whether the MI/PM2.5 association was modified by PM2.5 species mass fractions. Many studies have reported increased relative risks associated with primary PM, traffic sources, or markers of traffic pollution.23–25, 55,57–58 For example, Kim et al (2012) found increased ischemic heart disease admissions were associated with increased EC and OC concentrations in the previous day, but not with increased sulfate and nitrate concentrations.25 The one component that both primary and secondary PM have in common is organic matter.
Our study had several limitations that should be noted. First, we were only able to use central site PM2.5 mass concentrations, likely resulting in both Berkson and classical error,60,61 resulting in a bias towards the null, underestimating the risk of transmural MI associated with increased PM2.5 concentration. Second, we used daily PM2.5 species mass fractions averaged over 12 km by 12 km grids, and assigned them to each subject by residential location. This could have resulted in underestimates of concentrations of primary species (e.g., EC) for residences in close proximity to sources (e.g., < 200 m from a major roadway),45 which may have resulted in some error in placing subjects in high, middle, and low EC tertiles. However, this error is unlikely to cause a large number of subjects to be incorrectly placed in the ‘high’ EC tertile when in fact they should have been in the ‘low’ EC tertile, and vice-versa. Third, we were unable to examine trace elements (e.g. nickel, vanadium, aluminum, etc.) that have been either associated with increased risk of CV events directly, or shown to modify PM/CV associations.10, 12, 20, 21
We evaluated whether the relative odds of a transmural infarction associated with each 10.8 μg/m3 increase in PM2.5 concentration in the previous 24 hours was different when the mass fractions of sulfate, nitrate, ammonium, EC and OC on that day were high versus low. We found the largest relative odds estimates on the days with the highest tertile of sulfate, nitrate, and ammonium, and the lowest tertile of EC, suggesting these effects are greatest on days when the mixture is enhanced with secondary PM. Note, secondary species typically make up the bulk of PM2.5 in New Jersey.43 Further work is needed to investigate which secondary species (i.e. sulfate, nitrate, ammonium, secondary organic species formed through gas and/or aqueous chemistry, organosulfates, reactive species carried in aerosol water such as peroxides) is/are responsible for this finding.
Acknowledgments
We gratefully acknowledge Wyat Appel of EPA’s National Exposure Research Laboratory for his support with the application and description of the CMAQ model used in this work. This research was funded in part by the U.S. Environmental Protection Agency (Cooperative Agreement CR-83407201-0), NIEHS-sponsored UMDNJ Center for Environmental Exposures and Disease (NIEHS P30ES005022), and the New Jersey Agricultural Experiment Station. Barbara Turpin was supported, in part, by the U.S. Department of Agriculture NIFA. Natasha Hodas was supported by a Graduate Assistance in Areas of National Need (GAANN) Fellowship and an Environmental Protection Agency Science To Achieve Results Graduate Fellowship. Although this work was reviewed by EPA and approved for publications, it may not necessarily reflect official Agency policy.
References
- 1.Berglind N, Ljungman P, Moller J, Hallqvist J, Nyberg F, Rosenqvist M, Pershagen G, Bellander T. Air pollution exposure--a trigger for myocardial infarction? Int J Environ Res Public Health. 2010;7:1486–1499. doi: 10.3390/ijerph7041486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, Perucci CA. Air pollution and myocardial infarction in rome: A case-crossover analysis. Epidemiology. 2003;14:528–535. doi: 10.1097/01.ede.0000082046.22919.72. [DOI] [PubMed] [Google Scholar]
- 3.Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 4.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 5.Peters A, von Klot S, Heier M, Trentinaglia I, Cyrys J, Hormann A, Hauptmann M, Wichmann HE, Lowel H. Particulate air pollution and nonfatal cardiac events. Part I. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Res Rep Health Eff Inst. 2005:1–66. discussion 67–82, 141–148. [PubMed] [Google Scholar]
- 6.Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 7.Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB. Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ Health Perspect. 2010;118:1229–1234. doi: 10.1289/ehp.0901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: A multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16:41–48. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- 10.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179:1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, Dominici F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am J Epidemiol. 2008;168:1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19:680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 14.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long CM, Suh HH, Catalano PJ, Koutrakis P. Using time- and size-resolved particulate data to quantify indoor penetration and deposition behavior. Environ Sci Technol. 2001;35:2089–2099. doi: 10.1021/es001477d. [DOI] [PubMed] [Google Scholar]
- 16.Meng QY, Turpin BJ, Polidori A, Lee JH, Weisel C, Morandi M, Colome S, Stock T, Winer A, Zhang J. PM2.5 of ambient origin: Estimates and exposure errors relevant to PM epidemiology. Environ Sci Technol. 2005;39:5105–5112. doi: 10.1021/es048226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter LK, Ozkaynak H, Franklin M, Schultz BD, Neas LM. The use of improved exposure factors in the interpretation of fine particulate matter epidemiological results. Air Quality and Atmospheric Health. 2012 (in press) [Google Scholar]
- 18.Hodas N, Meng Q, Lunden MM, Rich DQ, Ozkaynak H, Baxter LK, Zhang Q, Turpin BJ. Variability in the fraction of ambient fine particulate matter found indoors and observed heterogeneity in health effect estimates. J Expo Sci Environ Epidemiol. 2012;22:448–454. doi: 10.1038/jes.2012.34. [DOI] [PubMed] [Google Scholar]
- 19.Rohr AC, Wyzga RE. Attributing health effects to individual particulate matter constituents. Atmospheric Environment. 2012;62:130–152. [Google Scholar]
- 20.Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in california: Results from calfine. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, Dominici F. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117:957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SY, Peel JL, Hannigan MP, Dutton SJ, Sheppard L, Clark ML, Vedal S. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120:1094–1099. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byun DS, Schere KL. Review of the governing equations, computational algorithms, and other components of the models-3 community multiscale air quality (CMAQ) modeling system. Applied Mechanics Reviews. 2006;59:51–77. [Google Scholar]
- 27.Kostis JB, Wilson AC, Lacy CR, Cosgrove NM, Ranjan R, Lawrence-Nelson J. Time trends in the occurrence and outcome of acute myocardial infarction and coronary heart disease death between 1986 and 1996 (a New Jersey statewide study) Am J Cardiol. 2001;88:837–841. doi: 10.1016/s0002-9149(01)01888-4. [DOI] [PubMed] [Google Scholar]
- 28.United States Environmental Protection Agency. Technology transfer network - AQS Datamart. Available: http://www.epa.gov/ttn/airs/aqsdatamart/index.html.
- 29.Steadman RG. The assessment of sultriness. Part II: Effects of wind, extra radiation and barometric pressure on apparent temperature. Journal of Applied Meteorology. 1979;18:874–885. [Google Scholar]
- 30.United States Environmental Protection Agency. Community Multiscale Air Quality Model. Available: http://www.epa.gov/AMD/CMAQ/cmaq_model.html.
- 31.Foley KM, Roselle SJ, Appel KW, Bhave PV, Pleim JE, Otte TL, Mathur R, Sarwar G, Young JO, Gilliam CG, Kelly JT, Gilliland AB, Bash JO. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. www.geosci-model-dev.net/3/205/2010/
- 32.Yarwood G, Roa S, Yocke M, Whitten G. Final report to the US EPA, rt-0400675. 2005. Updates to the carbon bond chemical mechanism. [Google Scholar]
- 33.Pleim JE. A combined local and nonlocal closure model for the atmospheric boundary layer. Part i: Model description and testing. Journal of Applied Meteorology and Climatology. 2007;46:1383–1395. [Google Scholar]
- 34.Pleim JE. A combined local and nonlocal closure model for the atmospheric boundary layer. Part ii: Application and evaluation in a mesoscale meteorological model. J Appl Meteor Clim. 2007;46:1396–1907. [Google Scholar]
- 35.Bey I, Jacob DJ, Yantosca RM, Logan JA, Field BD, Fiore AM, Li Q, Liu HY, Mickley LJ, Schultz MG. Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation. Journal of Geophysical Research. 2001;106:23,073–023,009. [Google Scholar]
- 36.Gilliland AB, Appel KW, Pinder R, Dennis RL. Seasonal nh3 emissions for the continental united states: Inverse model estimation and evaluation. Atmospheric Environment. 2006;40:4986–4998. [Google Scholar]
- 37.Appel K, Bhave P, Gilliland A, Sarwar G, Roselle S. Evaluation of the community multiscale air quality (cmaq) model version 4.5: Sensitivities impacting model performance; part ii - particulate matter. Atmospheric Environment. 2008:6054–6066. [Google Scholar]
- 38.Crooks JL, Özkaynak H. Simultaneous statistical bias correction of multiple PM2.5 species from a regional photochemical grid model. J Environmental and Ecological Statistics. 2012 (in press) [Google Scholar]
- 39.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Maclure M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 41.Reff A, Bhave PV, Simon H, Pace TG, Pouliot GA, Mobley JD, Houyoux M. Emissions inventory of PM2. 5 trace elements across the United States. Environ Sci Technol. 2009;43:5790–5796. doi: 10.1021/es802930x. [DOI] [PubMed] [Google Scholar]
- 42.McMurry P, Shepherd M, Vickery J, editors. NARSTO. Particulate Matter Science for Policy Makers: A NARSTO Assessment. Cambridge University Press; Cambridge, England: 2004. [Google Scholar]
- 43.U.S. EPA (U.S. Environmental Protection Agency) Integrated Science Assessment for Particulate Matter. Research Triangle Park, NC: National Center for Environmental Assessment, Office of Research and Development; 2009. [PubMed] [Google Scholar]
- 44.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. 2. John Wiley & Sons; 2006. ( http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_bookid=2126&VerticalID=0) [Google Scholar]
- 45.Polidori A, Kwon J, Turpin BJ, Weisel C. Source proximity and residential outdoor concentrations of PM(2.5), OC, EC, and PAHs. J Expo Sci Environ Epidemiol. 2010;20:457–468. doi: 10.1038/jes.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turpin BJ, Huntzicker JJ. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmospheric Environment. 1995;29:3527–3544. [Google Scholar]
- 47.Turpin BJ, Saxena P, Andrews E. Measuring and simulating particulate organics in the atmosphere: problems and prospects. Atmospheric Environment. 2000;34:2983–3013. [Google Scholar]
- 48.Hallquist MWJ, Baltensperger U. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmospheric Chemistry and Physics. 2009:3555–3762. [Google Scholar]
- 49.Turpin BJ, Saxena P, Andrews E. Measuring and simulating particulate organics in the atmosphere: Problems and prospects. Atmospheric Environment. 2000;34:2983–3013. [Google Scholar]
- 50.DeCarlo PFUI, Crounse J, de Foy B, Dunlea EJ, Aiken AC, Knapp D, Weinheimer AJ, Campos T, Wennberg PO, Jimenez JL. Investigation of the sources and processing of organic aerosol over the central mexican plateau from aircraft measurements during milagro. Atmos Chem Phys. 2010:5257–5280. [Google Scholar]
- 51.Ervens BTBWR. Secondary organic aerosol formation in cloud droplets and aqueous particles (AQSOA): A review of laboratory, field and model studies. Atmos Chem Phys Discuss. 2012;11:11069–11102. [Google Scholar]
- 52.Lanz VAAM, Baltensperger U, Buchmann B, Hueglin C, Prevot ASH. Source apportionment of submicron organic aerosols at an urban site by factor analysis modeling of apportionment of submicron organic aerosols at an urban site by factor analysis modeling of aerosol mass spectra. Atmos Chem Phys. 2007:1503–1522. [Google Scholar]
- 53.Yu JZ, Huang XF, Xu J, Hu M. When aerosol sulfate goes up, so does oxalate: Implication for the formation mechanisms of oxalate. Environ Sci Technol. 2005;39:128–133. [PubMed] [Google Scholar]
- 54.Surratt JD, Gomez-Gonzalez Y, Chan AW, Vermeylen R, Shahgholi M, Kleindienst TE, Edney EO, Offenberg JH, Lewandowski M, Jaoui M, Maenhaut W, Claeys M, Flagan RC, Seinfeld JH. Organosulfate formation in biogenic secondary organic aerosol. J Phys Chem A. 2008;112:8345–8378. doi: 10.1021/jp802310p. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Ito K, Lall R, Lippmann M, Thurston G. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect. 2011;119:461–466. doi: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. Cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 57.Institute HE. Traffic-related air pollution: A critical review of the literature on emissions, exposure, and health effects. 2010. pp. 1–386. [Google Scholar]
- 58.Ito K, Mathes R, Ross Z, Nadas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119:467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lall R, Ito K, Thurston G. Distributed lag analyses of daily hospital admissions and source-apportioned fine particle air pollution. Environ Health Perspect. 2011;119:455–460. doi: 10.1289/ehp.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bateson TF, Coull BA, Hubbell B, Ito K, Jerrett M, Lumley T, Thomas D, Vedal S, Ross M. Panel discussion review: Session three--issues involved in interpretation of epidemiologic analyses--statistical modeling. J Expo Sci Environ Epidemiol. 2007;17 (Suppl 2):S90–96. doi: 10.1038/sj.jes.7500631. [DOI] [PubMed] [Google Scholar]
- 61.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]