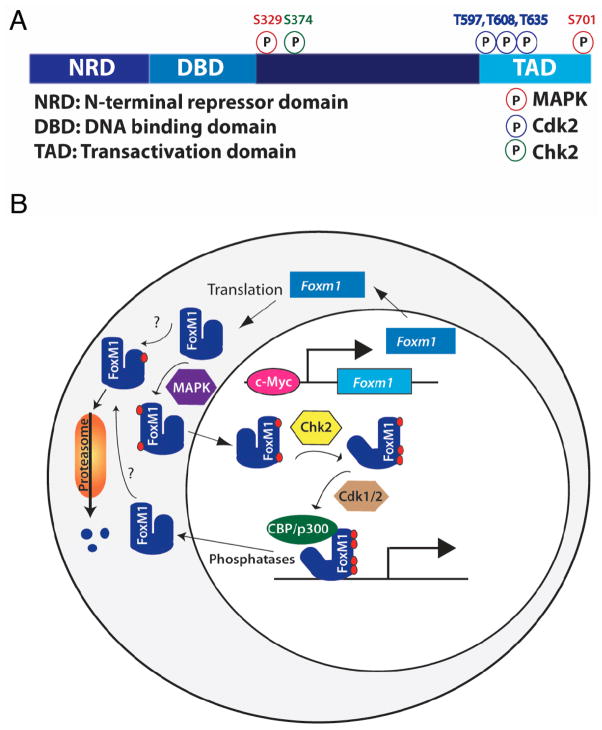
Fig. 2.
Model of FoxM1 regulation. A) FoxM1 contains an N-terminal repressor domain (NRD), a forkhead DNA binding domain, a transactivation domain (TAD), and a less-well characterized fourth region. Known mouse FoxM1 phosphorylation sites and their kinases are noted. B) Foxm1 expression and activity are tightly regulated. Foxm1 expression peaks in late G1, and is maintained through mitosis. FoxM1 activity is slightly delayed compared to its RNA expression, due to posttranslational regulation. FoxM1 cytoplasmic/nuclear shuttling is controlled by MAPK phosphorylation. Autorepression by the NRD is relieved by Chk2 phosphorylation, and the recruitment of coactivators is regulated by Cdk1/2 phosphorylation. Because Chk2 and Cdk1/2 are primarily nuclear proteins, phosphorylation of FoxM1 by these factors likely occurs in the nucleus, although this has not been confirmed. The order of phosphorylation events and whether multiple phosphorylation events are required for FoxM1 activity are currently unknown, and whether nuclear FoxM1 binds to DNA before phosphorylation by Chk2 and Cdk1/2 is unclear. N-terminal phosphorylation, dependent on the APC cofactor Cdh1, targets FoxM1 for proteosomal degradation. Phosphatases involved in FoxM1 dephosphorylation are currently unknown.