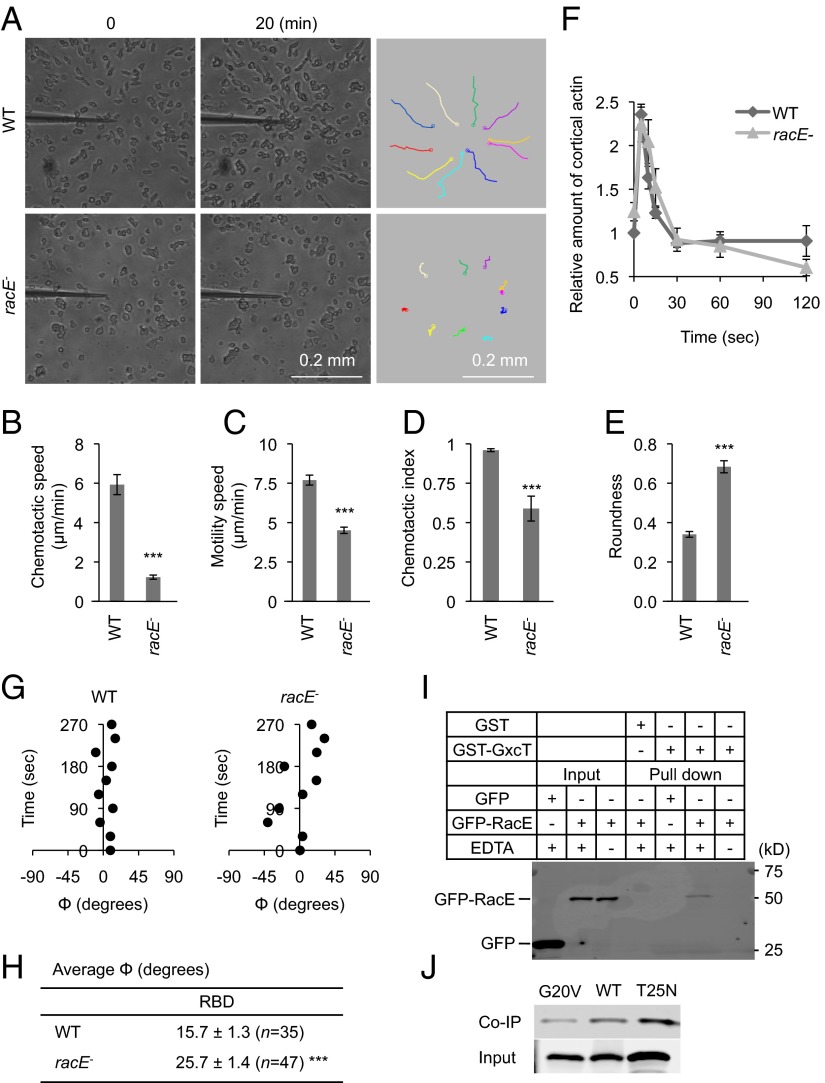
Fig. 6.
RacE is important for chemotaxis and binds to GxcT. (A) Developed WT and racE− cells were placed in a cAMP gradient and observed for 20 min. The trajectories of cell migration are shown (Right). (B–E) Chemotactic behaviors including chemotactic speed (B), motility speed (C), chemotactic index (D), and roundness (E) were quantified as described in Fig. 2 B–E. Values represent the mean ± SEM (n = 3). More than 20 cells were analyzed in each experiment. ***P < 0.001. (F) At the indicated time points after uniform cAMP stimulation, WT and racE− cells were lysed, and the cortical actin was isolated and quantified as described in Experimental Procedures. Values represent the mean ± SEM (n = 3). There are no significant differences between WT and racE− cells. (G) Time-lapse analyses of RBD-GFP localization were performed in the presence of a cAMP gradient and Latrunculin A. The position of the RBD-GFP crescents was quantified by calculating the angle Φ over time as described in Fig. 4 D–H. (H) The average value of Φ for RBD-GFP crescents was calculated from the time-lapse analyses in G. Values represent the mean ± SEM. N indicates the number of cells analyzed. ***P < 0.001. (I) Recombinant GST-GxcT was purified from E. coli and incubated, in the presence or absence of EDTA, with whole cell lysates prepared from Dictyostelium cells that were expressing either GFP-RacE or GFP. GST-GxcT was precipitated using glutathione beads, and the bound fraction was analyzed by immunoblotting using anti-GFP antibodies. GST was purified and precipitated as a negative control. (J) GST-GxcT was incubated with cell lysates expressing either GFP-RacE, GFP-RacE(G20V), or GFP-RacE(T25N) and precipitated using glutathione beads. The lysates (input) and bound fractions (coimmunoprecipitation, co-IP) were analyzed by immunoblotting using anti-GFP antibodies.