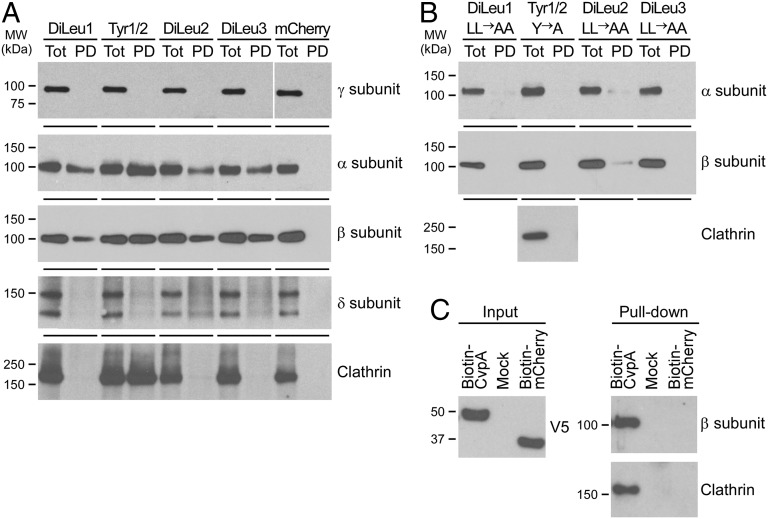
Fig. 5.
CvpA endocytic sorting motifs interact with AP2 and clathrin. (A) Pull-down assays using HeLa cell lysates and GST fusions to 30mer peptides containing the CvpA endocytic sorting motifs DiLeu1 (aa 2–31), DiLeu2 (aa 172–201), DiLeu3 (aa 299–328), or Tyr1/Tyr2 (aa 52–81). GST peptides and interacting proteins were collected with glutathione agarose beads and analyzed by immunoblot with antibodies against clathrin and clathrin adaptor complex subunits present in AP1 (subunit γ), AP2 (subunits α and β), or AP3 (subunit δ). Pull-down with GST-mCherry was used as a negative control. (B) Pull-down assays using GST fusions to 30mer peptides containing endocytic sorting motifs where diluecine residues were substituted with dialanines, and tyrosine residues were substituted with alanine. Immunoblotting was conducted with antibodies against the α- and β-subunits of AP2 or clathrin (Tyr1/Tyr2 pull-down). (C) Pull-down assay using biotinylated full-length CvpA. CvpA containing an N-terminal biotinylation signal and a C-terminal V5 tag was expressed in HeLa cells and then collected from cell lysates using streptavidin beads. Cells expressing biotin-mCherry or nontransfected cells (Mock) were used as negative controls. Immunoblot detection of the V5 tag was used to assess equal expression of biotin-tagged proteins (Left). Pull-down samples were immunoblotted with antibodies recognizing clathrin and the β-subunit of AP2 and clathrin (Right).