Significance
Intestinal epithelial barrier dysregulation is a hallmark of inflammatory bowel diseases (IBDs). A central role for hypoxic signaling has been defined in barrier modulation during inflammation. We demonstrate that genes involved in creatine metabolism, the creatine kinases (CKs), are coordinately regulated by hypoxia-inducible transcription factors (HIFs) and that such regulation is critical to barrier function. Inhibition of the CK pathway abrogates apical junction assembly and barrier integrity. Dietary creatine supplementation profoundly attenuates the pathogenic course of mucosal inflammation in mouse colitis models. Moreover, we demonstrate altered expression of mitochondrial and cytosolic CK enzymes in IBD patient tissue. These findings highlight the fundamental contribution of creatine metabolism to intestinal mucosal function, homeostasis, and disease resolution.
Keywords: epithelial junctions, energy metabolism, actomyosin, IBD
Abstract
Mucosal surfaces of the lower gastrointestinal tract are subject to frequent, pronounced fluctuations in oxygen tension, particularly during inflammation. Adaptive responses to hypoxia are orchestrated largely by the hypoxia-inducible transcription factors (HIFs). As HIF-1α and HIF-2α are coexpressed in mucosal epithelia that constitute the barrier between the lumen and the underlying immune milieu, we sought to define the discrete contribution of HIF-1 and HIF-2 transactivation pathways to intestinal epithelial cell homeostasis. The present study identifies creatine kinases (CKs), key metabolic enzymes for rapid ATP generation via the phosphocreatine–creatine kinase (PCr/CK) system, as a unique gene family that is coordinately regulated by HIF. Cytosolic CKs are expressed in a HIF-2–dependent manner in vitro and localize to apical intestinal epithelial cell adherens junctions, where they are critical for junction assembly and epithelial integrity. Supplementation with dietary creatine markedly ameliorated both disease severity and inflammatory responses in colitis models. Further, enzymes of the PCr/CK metabolic shuttle demonstrate dysregulated mucosal expression in a subset of ulcerative colitis and Crohn disease patients. These findings establish a role for HIF-regulated CK in epithelial homeostasis and reveal a fundamental link between cellular bioenergetics and mucosal barrier.
Intestinal epithelia function to both facilitate nutrient transport and protect against luminal antigens. Selective permeability is mediated by specialized anatomical features, including dynamic intercellular junctions. The apical junctional complex (AJC), comprising the tight junction (TJ) and subjacent adherens junction (AJ), is supported by a highly crosslinked cytoskeleton and is the key determinant of paracellular permeability and barrier function. Prominent features of the perijunctional cytoskeleton include an extensive network of F-actin bundles that associate with TJs, and a dense circumferential ring of actin and myosin contiguous with AJs (1). This actomyosin ring readily copurifies with other cytoskeletal proteins and demonstrates ATP-dependent contractility ex vivo. Such observations highlight the contractile nature of the apical actin network and the intimate association between its components (2).
Based on their juxtaposition to the anoxic gut lumen, intestinal epithelial cells (IECs) function physiologically in a low-oxygen-tension microenvironment and exhibit a uniquely adaptive oxygenation profile. This profile is prodigiously altered in inflammatory bowel disease (IBD) (3). Adaptive transcriptional responses to oxygen deprivation are mediated primarily through the hypoxia-inducible factor (HIF) complex, comprising a constitutive “β” subunit, and an oxygen-labile “α” component that is regulated in part by prolyl hydroxylase (PHD) enzymes (4). Despite their concurrent expression in many cell types, HIF-1α and HIF-2α play nonredundant roles (5) that appear to be highly cell specific to facilitate both short- and long-term adaptations to hypoxia (6).
Barrier dysregulation with unimpeded flux of luminal antigens contributes fundamentally to the profound metabolic shifts inherent to mucosal inflammatory lesions. Both HIF-1α and HIF-2α are expressed in inflamed mucosa from IBD patients (7) and mouse models of colitis (8). Studies of murine IBD have revealed that loss of epithelial HIF-1α correlates with more severe clinical symptoms, whereas constitutive activation of HIF-1 and HIF-2 is protective (8). Despite this, the molecular targets of HIF-1 and particularly HIF-2 in IECs are not well characterized. To delineate HIF-1– and HIF-2–specific target loci, we performed ChIP–chip analysis of chromatin isolated from hypoxic IECs. We identified a family of genes involved in creatine (Cr) metabolism that are coordinately regulated by HIF-2. Immunolocalization of the cytosolic “muscle-” and “brain-type” creatine kinases (CKM and CKB, respectively) revealed coupling to the AJC, and CK inhibition abrogated junctional assembly and barrier function. Moreover, dietary Cr supplementation ameliorated the pathogenic course of murine colitis. Collectively, these data identify a unique role for HIF in modulating the epithelial barrier through regulation of the Cr/CK shuttle.
Results
HIF ChIP-on-Chip Analysis Identifies Genes Involved in Cr Metabolism.
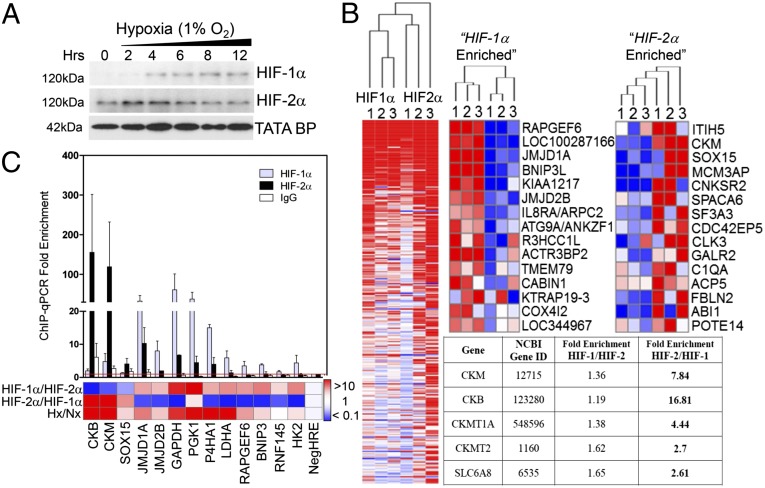
ChIP was performed in human colon carcinoma Caco-2 IECs using HIF-1α– and HIF-2α–specific polyclonal antibodies (Fig. 1A). ChIP-enriched and input DNA were hybridized to a custom microarray comprising a genome-wide set of predicted transcription start site (TSS) flanking sequences at 50 bp resolution. Consistent with previous reports (5), positive hits included cohorts enriched for both HIF-1 and HIF-2 binding, as well as loci unique for HIF-1 or HIF-2 occupancy alone (Fig. 1B). Bioinformatic analyses for de novo motifs (MEME) and transcription factor binding sites (9) identified the canonical hypoxia response element (HRE) motif 5′-RCGTG-3′ as a highly represented consensus sequence. Independent ChIP–quantitative PCR (qPCR) analyses confirmed enrichment at discrete loci compared with IgG control, with all loci displaying a threshold of twofold enrichment in hypoxia relative to an HRE-negative region. Importantly, integration of ChIP–chip and ChIP–qPCR analyses confirmed robust HIF-1α enrichment of established HIF-1 targets (Fig. 1C).
Fig. 1.
HIF-1 and HIF-2 ChIP-on-chip. (A) Accumulation of nuclear HIF-1α and HIF-2α in Caco-2 IECs in normoxia (0 h) and hypoxia. (B) Heat maps generated from normalized smoothed ratios of ChIP enrichment (GENE-E), showing common targets (Left) and discrete promoter occupancy (Right). (Inset) Functional annotation clustering of ChIP–chip hits revealed genes involved in Cr metabolism as HIF-2–specific targets (C) ChIP–chip results were validated by qPCR analysis following ChIP using anti–HIF-1α, anti–HIF-2α, or IgG. Bar graph represents fold enrichment of ChIP DNA compared with input chromatin (n = 3).
In the course of this analysis, we identified the CK pathway as a unique target of HIF-2. Indeed, CKM featured as one of the most significantly enriched HIF-2α–specific loci. Further interrogation of this chip cluster revealed additional genes implicated in Cr metabolism, namely CKB, mitochondrial CK (CKMT1), and the major Cr transporter (SLC6A8) (Fig. 1B, Table S1). The CK shuttle defines a key metabolic pathway for temporal and spatial energy buffering. CK regulates cellular ATP through the reversible transfer of high-energy phosphate from phospho-Cr (PCr), connecting sites of ATP generation with compartmentalized ATP utilization (10). ChIP–qPCR quantitation of HIF binding to promoter regions of CKB and CKM revealed specific occupancy of HIF-2 over HIF-1, with increased enrichment in hypoxia (Fig. 1C).
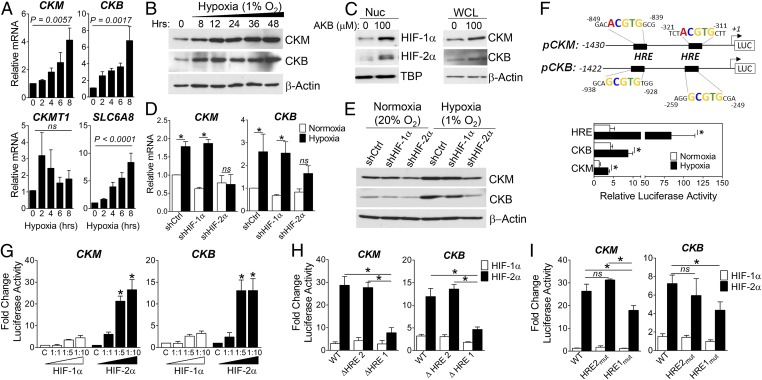
To investigate correlation of HIF-2 binding to hypoxia-induced gene expression, we evaluated expression of Cr metabolic genes by qPCR. Transcript levels of Cr transporter (SLC6A8) and cytosolic (CKM, CKB) CK isozymes were increased in a time-dependent manner in hypoxia (Fig. 2A). CK protein levels were similarly increased both in hypoxic Caco-2 lysates (Fig. 2B) and in cells treated with the PHD inhibitor AKB to stabilize HIF-1α and HIF-2α protein under normoxic conditions (Fig. 2C). These data indicate that CK is induced in IECs in response to both hypoxia and pharmacologic HIF stabilization, consistent with HIF-mediated transcription.
Fig. 2.
HIF-2–dependent up-regulation of CK enzymes. (A) qPCR analysis of CK enzyme (CKM, CKB, CKMT1A) and Cr transporter (SLC6A8) expression in Caco-2 IECs (20% or 1% O2, 6 h; n = 4). (B) Immunoblot analysis of CK levels in Caco-2 IECs (1% O2). (C) Immunoblot analysis of nuclear (HIF) and whole-cell lysates (CK) from Caco-2 treated with vehicle (DMSO) or PHD inhibitor (AKB; 100 μM) for 24 h. (D) qPCR analysis of CK expression in shCtrl, shHIF-1α, or shHIF-2α Caco-2 (20% or 1% O2, 6 h; n = 4) *P < 0.05. (E) Immunoblot analysis of CK levels in shCtrl, shHIF-1α, and shHIF-2α IECs (20% or 1% O2, 24 h). (F, Schematic) Proximal promoter of human CKM and CKB; TSS designated +1. T84 cells were nucleofected (24 h) and cultured in 20% or 1% O2 for 24 h. Data represent ratio of promoter-luc:pRen-luc activity (n = 4; *P < 0.05). (G) Caco-2 IECs were cotransfected with pCK-Luc and pcDNA3.1 vector as control (C) or increasing ratios of pcDNA3.1–HIF-1α∆ODD or pcDNA3.1–HIF-2α∆ODD (n = 3; *P < 0.0001). (H and I) Luciferase activity in lysates cotransfected with full-length (WT), truncated pCK-Luc (schematic) (n = 3; *P < 0.0001), or pCK-Luc harboring mutations in distal (HRE2) and proximal (HRE1) sites, and HIF plasmids at a 1:5 ratio (n = 4; *P < 0.05 by ANOVA).
CK Enzymes Are Induced in Hypoxic IECs in a HIF-2–Dependent Manner.
To determine the specificity of HIF-induced CK expression, we used short hairpin RNA (shRNA) knockdown to individually deplete HIF-1α and HIF-2α (Fig. S1A). qPCR analyses demonstrated that CK mRNA levels were significantly induced by 6 h in response to hypoxia in sh control (shCtrl) and HIF-1α knockdown cells, but not in cells with specific depletion of HIF-2α (Fig. 2D; P < 0.05). Moreover, knockdown of HIF-2α selectively attenuated basal levels and hypoxic induction of CK protein (Fig. 2E).
Analysis of human CKM and CKB TSS-flanking gene sequences revealed two candidate HRE sites in each promoter (Fig. 2F). To complement our ChIP–qPCR analysis (Fig. 1C), 1.4 kb fragments of CK promoter sequences were cloned upstream of a luciferase reporter. T84 IECs were nucleofected with either pCK- or pHRE-Luc reporter constructs, and exposed to 20% or 1% O2 for 24 h. For each construct analyzed, increased promoter activity was observed in hypoxia (Fig. 2F). To distinguish between HIF-1α– and HIF-2α–mediated influences, pCK-Luc was cotransfected into Caco-2 cells with increasing concentrations of oxygen-stable HIF-α expression vectors. Dose-dependent induction of pCK-Luc was observed with HIF-2α, but not HIF-1α, consistent with ChIP–qPCR and shHIF-2α findings (Fig. 2G). Promoter activity was significantly diminished upon deletion of the proximal HRE (HRE1) in both constructs (Fig. 2H). Mutational analysis revealed selective repression of HIF-2–regulated promoter activity upon mutation of HRE1 in pCK-Luc (Fig. 2I). Taken together, these findings indicate that HIF-2 specifically binds to proximal HRE sites in the promoter region of CK genes to directly activate their transcription in IECs.
Cytosolic CK Is Localized to Apical AJs.
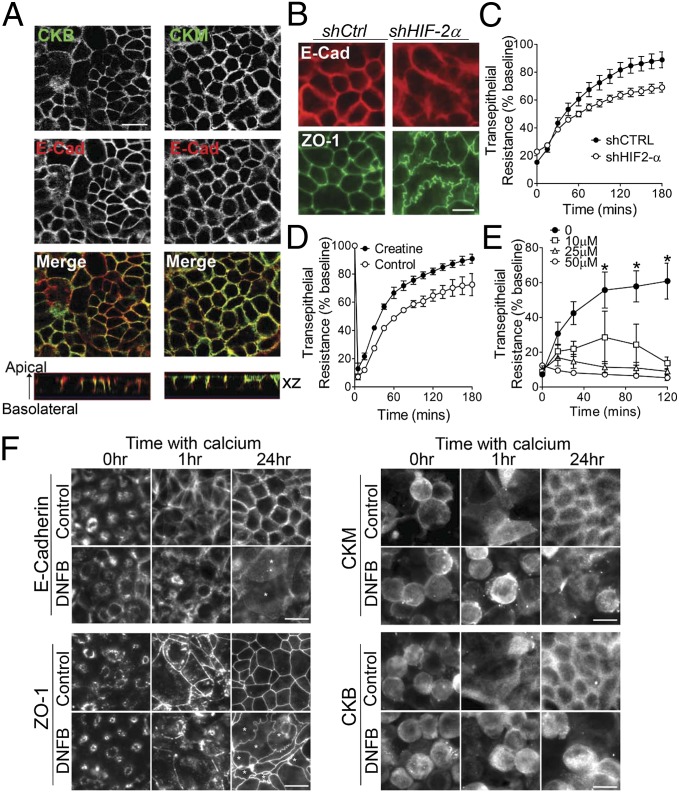
Differentially localized CK isozymes facilitate a high-energy PCr/CK circuit in polarized cells, wherein mitochondria are located distantly from regions of ATP consumption (10). Previous studies defined CKB and mitochondrial CK as distinct terminals of this circuit in IECs (11, 12). Functional coupling between CKB and myosin II (MII) at the circumferential ring is proposed to confer a spatial energetic advantage for myosin ATPase activity. MII isoforms localize to cadherin junctions, where they regulate AJ development and force generation through diverse mechanisms (13–15). In polarized IEC, both MIIA and cytosolic CKs colocalized with actin and E-cadherin at apical junctions (Fig. S2 A and B), and coimmunoprecipitation analyses revealed enrichment of MII in fractions precipitated by an anti-CK antibody (Fig. S2C). Detailed optical section analysis revealed that CKM and CKB are highly enriched at AJs, with further distribution of CKM throughout the lateral membrane (Fig. 3A).
Fig. 3.
CK contributes to apical junction assembly. (A) Caco-2 cells were stained for CKB, CKM, and E-cadherin. (Scale bar, 20 μm.) (B) Lateral membrane boundaries in Caco-2 shCtrl and shHIF-2α cells stained for E-cadherin and ZO-1. (Scale bar, 10 μm.) (C) shCtrl and HIF-2α–depleted T84 cells on permeable inserts were treated with 2 mM EDTA for 5 min and switched to HBSS with normal Ca2+ (1.8 mM). TER was measured over time; data represent percent recovery over time relative to baseline values (n = 3, P < 0.0001 by ANOVA). (D) T84 cells preloaded with 10 mM Cr monohydrate were subjected to Ca2+ switch, and TER measured over time (n = 3, P < 0.0001 by ANOVA). (E) T84 cells were subjected to Ca2+ switch in the presence of CK inhibitor DNFB. TER was monitored over time (data relative to no DNFB; n = 3, *P < 0.05). (F) T84 IECs incubated in low Ca2+ media (16 h) before switching to normal Ca2+, with or without 10 μM DNFB, were stained for E-cadherin, ZO-1, CKM, and CKB. (Scale bar, 10 μm.)
Intercellular actomyosin forces that support mature apical junctions are transmitted through AJs associated with actin filaments (16). Moreover, TJ assembly and sealing of the paracellular space is AJ-dependent. Given that CKs accumulate at AJs, functionally support MII ATPase, and represent HIF-2 target genes, we speculated that apical junctions may be altered in the absence of HIF-2 signaling. Localization of E-cadherin and ZO-1 in shCtrl monolayers revealed linear contours of apical lateral membranes (Fig. 3B). In contrast, HIF-2α–depleted cells displayed nonuniform, undulating junctions, consistent with attenuated intercellular tension and junction formation (15). Transepithelial electrical resistance (TER) measurements further indicated abrogated barrier function (57% ± 3.5%) of HIF-2α–depleted IECs compared with control monolayers (Fig. S3B). Moreover, assessment of TER development following Ca2+ switch assay revealed that barrier formation of HIF-2α knockdown IECs was significantly attenuated compared with control cells (Fig. 3C).
CK Contributes to Junctional Assembly After Ca2+ Switch.
To directly assess the contribution of CK to AJC assembly, we monitored the influence of altered CK signaling on TER development following Ca2+ switch. Cr supplementation markedly enhanced barrier recovery (Fig. 3C), whereas inhibition of CK using dinitrofluorobenzene (DNFB) (17) impaired recovery in a dose-dependent manner (Fig. 3D). CK inhibition correlated with a reduction in Cr and PCr metabolite levels, and increased IEC Cr/PCr ratio (Fig. S3A). To evaluate junction assembly dynamics, IECs were cultured overnight in low-Ca2+ media to permit cellular depolarization and protein translocation. As previously characterized (18), E-cadherin and ZO-1 localized to subapical ring-like structures upon extended Ca2+ depletion (Fig. 3F), whereas CK labeling was observed throughout the cytoplasm. One hour post-Ca2+ repletion, immunolabeling revealed nascent AJs and initiation of TJ assembly in control cells. Junctional assembly was markedly retarded in cells treated with DNFB. By 24 h, control monolayers assumed “mature” AJC lateral membrane staining. Strikingly, CK inhibition resulted in an undulating junctional staining pattern reminiscent of HIF-2α–depleted cells (Fig. 3B). Previous studies have shown that a basal level of MII phosphorylation and activity is necessary for apical junction integrity (13, 19). As such, HIF-mediated induction of CK isozymes might temporally buffer PCr supply and ATPase activity during periods of energetic stress. To test this hypothesis, we exposed IECs to 1% O2 in the presence or absence of DNFB. CK inhibition markedly decreased TERs in T84 monolayers exposed to 1% O2 (Fig. S3; P < 0.01), indicating that barrier function is compromised under hypoxic conditions upon inhibition of the CK/PCr shuttle.
Loss of HIF Signaling Results in Altered CK/PCr Circuit in IECs and Potentiates Murine Colitis.
To validate the regulation of CK by HIF signaling in vivo, we evaluated expression of Ckb in IECs derived from Hif-1β IEC-specific knockout mice (Fig. S4A; Hif-1β−/−) compared with WT (Hif-1β+/+) littermates. As outlined in Fig. S4B, Ckb expression was repressed (51.5% ± 5.6%) in Hif-1β−/− IECs, whereas levels of Pgk1, a HIF-1 target gene, were repressed by 29% ± 6.5%. Analysis of Cr and PCr metabolite levels in epithelial isolates revealed a significant increase in the Cr/PCr ratio of Hif-1β−/− IECs (Fig. S4C), consistent with CK inhibition in vitro (Fig. S3A). As an important correlative, we used the dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzene sulfonic acid (TNBS) colitis models to ascertain the effect of altered CK metabolism on IEC permeability. Acute DSS resulted in significantly increased intestinal permeability in Hif-1β−/− mice compared with Hif-1β+/+ littermate controls, as determined by translocation of FITC–dextran into serum post–oral administration (Fig. S4D). Similarly, Hif-1β−/− mice demonstrated increased colitic disease activity in response to TNBS, resulting in attenuated survival (Fig. S4E), augmented colonic shortening (Fig. S4F), and increased serum FITC–dextran compared with Hif-1β+/+ controls (Fig. S4G; 4.4- ± 1.6-fold; P = 0.0225). Further, increased inflammatory cytokine expression was determined in Hif-1β+/+ mice versus controls (Fig. S4H). Collectively, these findings indicate that IEC Hif signaling mediates a protective barrier effect in these IBD models (8).
Cr Supplementation Attenuates the Severity of Murine Colitis.
Barrier maintenance requires an intricate balance between AJC and cytoskeletal rearrangements that facilitate continual IEC turnover and transepithelial transport, both energy-dependent processes. Reduced ATP levels have been observed in inflamed IBD biopsies (20), and noninflamed tissues from Crohn disease (CD) patients are more sensitive to uncoupling of oxidative phosphorylation (21). Dietary Cr supplementation has been shown to confer protection in multiple disease models that display bioenergetic dysregulation (22, 23). We thus sought to evaluate the influence of Cr supplementation on mucosal inflammatory pathogenesis in murine models of IBD.
Mice were fed either normal chow or chow supplemented with 2% Cr (23), and exposed to 3% (wt/vol) DSS in drinking water. Cr-supplemented animals exhibited markedly reduced susceptibility to DSS-induced colitis, demonstrated by attenuated weight loss and colon shortening (Fig. S5 A and B). Histologic examination of control Cr-fed mouse colons revealed no overt alterations, confirmed by blinded scoring (Fig. S5 C and D). DSS-induced colitis in chow-fed animals resulted in extensive crypt loss, epithelial damage (Fig. S5E) and inflammatory cell infiltrate, as well as loss of solid stool (Fig. S5E). Interestingly, Cr supplementation attenuated histologic indices and promoted fecal pellet formation. Compared with 2% Cr-fed mice, chow-fed DSS mice displayed increased intestinal permeability (Fig. S5F). Consistent with this, serum and colonic levels of proinflammatory mediators were reduced in mice fed 2% Cr compared with chow alone (Fig. S6 A and B). HPLC analysis revealed increased colonic Cr in both Cr-fed vehicle and DSS-challenged mice compared with chow-fed cohorts (Fig. S6C). Interestingly, DSS challenge itself resulted in elevated Cr levels, indicating that Cr metabolism is altered in acute mucosal inflammation.
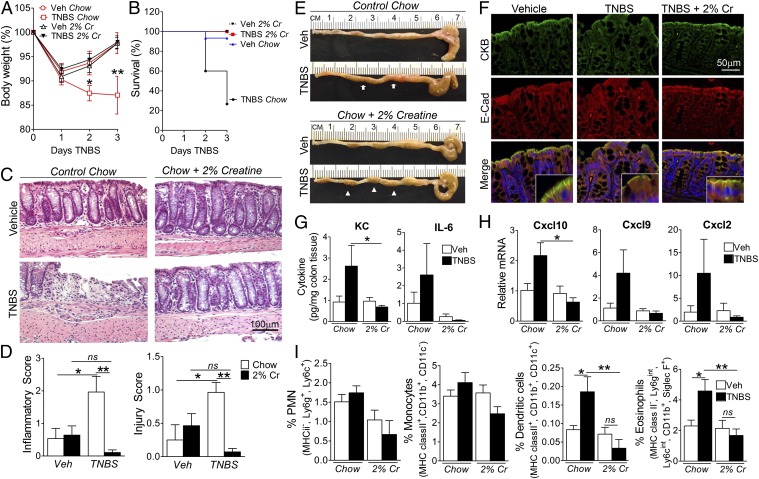
We next extended these findings to the independent TNBS colitis model. Within 2 d of TNBS challenge, chow-fed mice displayed progressive weight loss relative to vehicles (Fig. 4A; P < 0.05). Strikingly, Cr supplementation rendered mice refractory to TNBS-induced weight loss. To determine whether attenuated wasting translates to a survival benefit in Cr-fed mice, we recorded mortality following TNBS challenge. Cr supplementation resulted in enhanced survival over the colitic course (Fig. 4B). Histologic analysis of colon biopsies (Fig. 4C) revealed significant protection conferred by Cr on both injury and inflammatory indices (Fig. 4D), which correlated with fecal pellet formation (Fig. 4E). Vehicle IECs displayed apical enrichment of CKB that colocalized with E-cadherin (Fig. 4F). With TNBS challenge in chow-fed animals, patchy disruption of epithelial and crypt architecture associated with loss of E-cadherin junctional staining and colocalization with CKB. In contrast, both IEC integrity and junctional staining was preserved in Cr-fed mice exposed to TNBS. To ascertain whether epithelial integrity correlates with abrogated inflammatory stimuli, we profiled inflammatory mediators and lamina propria leukocyte populations (Fig. S7). Cr supplementation reduced colonic expression of proinflammatory chemokines compared with chow-fed mice (Fig. 4 G and H). Moreover, we identified a significant reduction in both dendritic (P < 0.001) and eosinophil (P < 0.01) populations in Cr-treated versus chow-fed TNBS mice (Fig. 4I). Taken together, these findings strongly imply that Cr supplementation exerts a beneficial effect in colitis-associated mucosal inflammation.
Fig. 4.
Cr is protective in TNBS-induced colitis. (A) Mice (n = 15 per group) were fed regular or 2% Cr-supplemented chow for 3 wk before EtOH (Veh) or TNBS administration. Change in body weight was determined (*P < 0.05; **P < 0.001). (B) Kaplan–Meier curve showing survival of chow and 2% Cr-fed mice (log-rank statistic = 18.47; P < 0.0001). (C and D) Histologic sections of colons were scored blindly for pathology indices. (Scale bar, 100 μm.) *P < 0.05; **P < 0.01. (E) Colon anatomy 3 d post-TNBS. Arrows indicate loose stool content in chow-fed mice; arrowheads indicate normal fecal pellet formation in 2% Cr-fed colon. (F) Colonic sections immunostained for CKB and E-cadherin. (Scale bar, 50 μm.) (G) Whole colon KC and IL-6 concentrations (*P < 0.05 by ANOVA, n = 5 mice per group). (H) Colonic chemokine mRNA levels (*P < 0.05 by ANOVA; n = 5 mice per group). (I) Colonic leukocyte populations [neutrophils/polymorphonuclear cells (PMN), monocytes, dendritic cells, eosinophils] assessed by flow cytometric analysis (n = 10–15; *P < 0.05; **P < 0.01 by ANOVA).
Attenuated CK Levels Suggest Altered Cr Signaling in Intestinal Biopsies from IBD Patients.
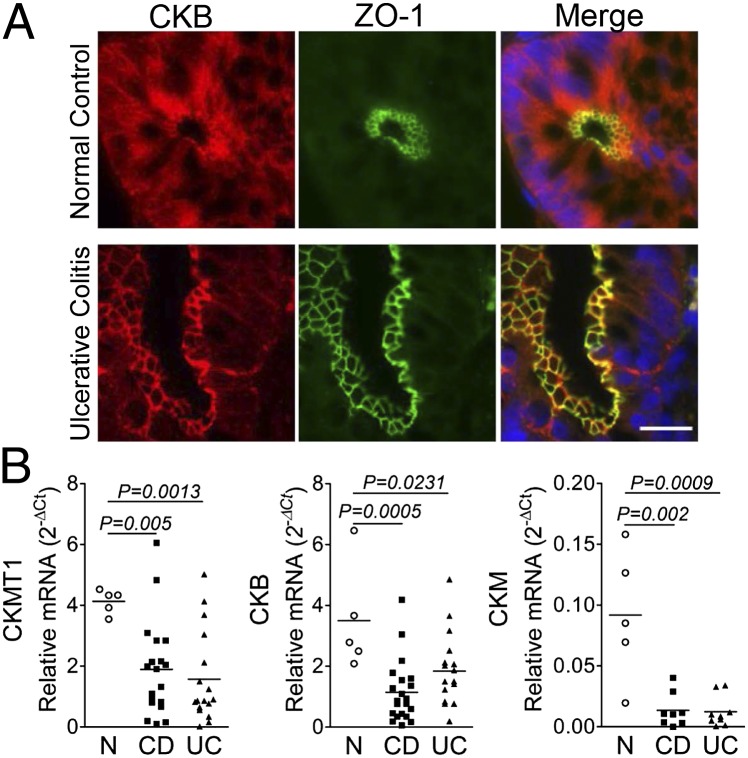
To investigate the relevance of our findings to human disease, we stained archived paraffin-embedded colon biopsy sections from IBD patients with antibodies to CKB and ZO-1. Similar to murine colonic sections, CKB exhibited diffuse cytoplasmic staining in non-IBD tissue, with enrichment at apical junctions colocalized with ZO-1 (Fig. 5A). Interestingly, in colon biopsies from IBD patients, CKB displayed preferential junctional staining relative to cytoplasmic compartments (Fig. 5A). As a corollary, we compared expression of CK enzymes in intestinal biopsies from IBD and non-IBD subjects. A significant decrease in transcript levels of CKM, CKB, and CKMT1 was observed in IBD patient tissue compared with non-IBD controls (Fig. 5B). These observations implicate marked alterations in the CK/PCr energy circuit in chronic human mucosal inflammation.
Fig. 5.
CK expression is reduced in chronic IBD. (A) Representative image of colon biopsy from a non-IBD control (Upper) and ulcerative colitis (UC) patient (Lower) immunostained for CKB and apical TJ marker ZO-1. (Scale bar, 20 μm.) (B) Transcript levels of CKM, CKB, and CKMT1A assessed by qPCR in cDNA derived from non-IBD control (n = 5), CD (n = 21), and UC (n = 17) individuals. Data expressed as relative Ct values, calculated as 2-∆Ct (Ct target – Ct actin) and analyzed by ANOVA.
Discussion
The cytoskeletal network that supports apical IEC junctions is among the most highly ordered arrays of actin filaments in nature (24). The circumferential actomyosin ring mediates selective barrier function in both health and disease (25), and is a primary target for molecular remodeling by diverse inflammatory stimuli (26). Moreover, actomyosin contraction is central to homeostatic and pathologic IEC shedding and barrier restitution (27). Epithelial function and barrier integrity is further regulated by low oxygen tension, through HIF-elicited adaptive pathways (28, 29). Notable for the present work, a recent study highlighted a role for epithelial Hif-2α in mediating proinflammatory epithelial responses in murine models of chemical and bacterial colitis, in contrast to the barrier protective program elicited by Hif-1α (30). As epithelial Hif1signaling has also been shown to promote inflammation in one study using DSS colitis (31), such findings likely reflect the context-dependent nature of these models. Indeed, the temporal kinetics of HIF-1α and HIF-2α in IBD appear to be different, with HIF-1α stabilized earlier in the disease course (as early as day 1 in TNBS colitis) (8) and a more prominent HIF-2α signal at later time points (by day 3 in DSS colitis) (30). Nonetheless, head-to-head comparisons have yet to be done, and the relative contribution of HIF-1 versus HIF-2 to mucosal signaling defines a key question. In the current work, we identify CK as a unique HIF target, and define a role for the CK energy shuttle in AJC assembly and epithelial homeostasis.
Initial studies of the IEC CK shuttle demonstrated functional coupling between CKB and myosin in isolated brush borders, specifically at the circumferential actomyosin ring (11, 12). MII is the major IEC cytoskeletal motor that converts ATP hydrolysis into mechanical forces, mediating the static tension and contractility of actin filaments. Interestingly, isoform-specific knockdown revealed differential roles for MIIA and MIIB in coordinating E-cadherin–based intercellular junction dynamics (14). Our observations indicate that CKs localize to apical AJs, where they likely constitute one terminal of a functional PCr/CK energy circuit that supplies energy to myosin motors. In addition to PCr generation via oxidative phosphorylation coupling, a subset of cytosolic CK has been shown to associate with glycolytic enzymes to facilitate PCr repletion, wherein HIF-1–mediated induction of the glycolytic pathway for ATP generation represents a central adaptation for cell survival (10). Given the importance of junction integrity to epithelial homeostasis, apical ATPases associated with the AJC seem likely candidates for prioritized PCr targeting under conditions of energetic stress. Coupled with our findings, this posits an intriguing link between HIF signaling and a unique role for Cr/PCr in junctional energetics. This complex metabolic regulation underscores the importance of balancing ATP supply and consumption in cells with high and fluctuating energy demands.
In the present studies, Cr supplementation was shown to provide substantial benefits in murine colitis. This was mainly attributable to enhanced cellular energetics through the PCr/CK circuit, but may also be explained in part by Cr-mediated reduction of oxidative stress (10). Cr has been shown to possess antioxidant properties (32), whereas mtCK activation was found to reduce mitochondrial reactive oxygen species (ROS) (33). These properties define an intriguing overlap with HIF-2 transcriptional programs, as HIF-2 regulates expression of a number of antioxidant enzymes (34). Furthermore, loss of HIF-2α results in increased ROS and redox imbalance in multiple cellular settings. That HIF-mediated induction of CK may represent an antioxidant pathway to buffer redox homeostasis in hypoxic cells certainly warrants further investigation.
Finally, our data demonstrating attenuated expression of CK enzymes in IBD tissue suggest that intestinal Cr metabolism and PCr/CK energetics may be compromised in at least a subset of IBD patients. This observation is noteworthy on two levels. First, bioenergetic dysregulation resulting from impaired CK shuttling may contribute to the increased barrier permeability characteristic of inflamed mucosae (25). Second, chronic inflammation associated with IBD is a major risk factor for colitis-associated cancer. A number of studies have now identified reduced levels of CKB in colonic tumors (35, 36), whereas studies using dominant negative CKB mutants have defined a correlation with molecular markers of epithelial-to-mesenchymal transition in colon cancer cells (37). Taken together, these observations provide a compelling argument for Cr supplementation as an adjuvant therapy to promote epithelial restitution and ameliorate mucosal inflammation via enhanced cellular energetics.
Materials and Methods
Please refer to SI Materials and Methods for detailed descriptions of the methods.
Animal Studies.
Colitis models were induced as described in SI Materials and Methods.
ChIP–Chip.
Input and HIF ChIP-DNA were hybridized to a custom microarray designed to cover a genome-wide set of human promoter regions of ≤2 kb (Switchgear Genomics). All microarray data have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus (GSE43108).
Supplementary Material
Acknowledgments
We gratefully acknowledge Frank Gonzalez (National Cancer Institute) for his kind donation of the Hif-1β flox mice. This work was supported by National Institutes of Health Grants DK50189, HL60569, and DK095491, and by the Crohn’s and Colitis Foundation of America.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE43108).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302840110/-/DCSupplemental.
References
- 1.Hirokawa N, Keller TC, 3rd, Chasan R, Mooseker MS. Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J Cell Biol. 1983;96(5):1325–1336. doi: 10.1083/jcb.96.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177(2):512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colgan SP, Taylor CT. Hypoxia: An alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7(5):281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 5.Ratcliffe PJ. HIF-1 and HIF-2: Working alone or together in hypoxia? J Clin Invest. 2007;117(4):862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giatromanolaki A, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56(3):209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114(8):1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartharius K, et al. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 10.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon PV, Keller TC., 3rd Functional coupling to brush border creatine kinase imparts a selective energetic advantage to contractile ring myosin in intestinal epithelial cells. Cell Motil Cytoskeleton. 1992;21(1):38–44. doi: 10.1002/cm.970210105. [DOI] [PubMed] [Google Scholar]
- 12.Keller TC, 3rd, Gordon PV. Discrete subcellular localization of a cytoplasmic and a mitochondrial isozyme of creatine kinase in intestinal epithelial cells. Cell Motil Cytoskeleton. 1991;19(3):169–179. doi: 10.1002/cm.970190305. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov AI, et al. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE. 2007;2(7):e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smutny M, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12(7):696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 16.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8(8):633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 17.Linton JD, et al. Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci USA. 2010;107(19):8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16(6):2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15(6):2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schürmann G, et al. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis. 1999;14(1):41–46. doi: 10.1007/s003840050181. [DOI] [PubMed] [Google Scholar]
- 21.Söderholm JD, et al. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut. 2002;50(3):307–313. doi: 10.1136/gut.50.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klivenyi P, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5(3):347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 23.Lin YS, et al. Dysregulated brain creatine kinase is associated with hearing impairment in mouse models of Huntington disease. J Clin Invest. 2011;121(4):1519–1523. doi: 10.1172/JCI43220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 25.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 26.Koch S, Nusrat A. Dynamic regulation of epithelial cell fate and barrier function by intercellular junctions. Ann N Y Acad Sci. 2009;1165:220–227. doi: 10.1111/j.1749-6632.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 27.Marchiando AM, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140(4):1208–1218. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140(6):1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastrogiannaki M, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue X, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145(4):831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah YM, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134(7):2036–2048. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun. 2002;290(1):47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 33.Meyer LE, et al. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem. 2006;281(49):37361–37371. doi: 10.1074/jbc.M604123200. [DOI] [PubMed] [Google Scholar]
- 34.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35(4):331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 35.Balasubramani M, Day BW, Schoen RE, Getzenberg RH. Altered expression and localization of creatine kinase B, heterogeneous nuclear ribonucleoprotein F, and high mobility group box 1 protein in the nuclear matrix associated with colon cancer. Cancer Res. 2006;66(2):763–769. doi: 10.1158/0008-5472.CAN-05-3771. [DOI] [PubMed] [Google Scholar]
- 36.Friedman DB, et al. Proteome analysis of human colon cancer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 2004;4(3):793–811. doi: 10.1002/pmic.200300635. [DOI] [PubMed] [Google Scholar]
- 37.Mooney SM, et al. Creatine kinase brain overexpression protects colorectal cells from various metabolic and non-metabolic stresses. J Cell Biochem. 2011;112(4):1066–1075. doi: 10.1002/jcb.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.