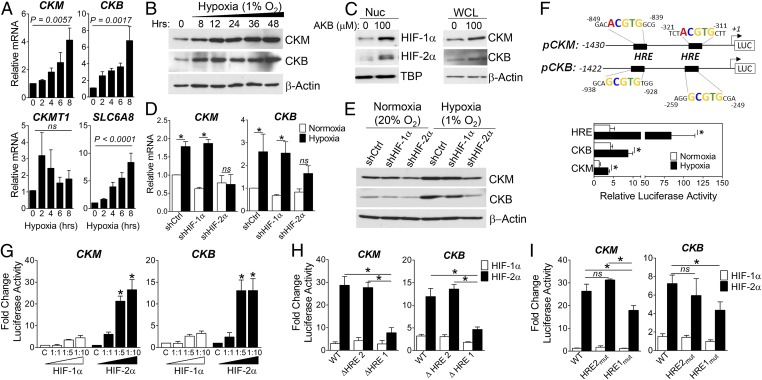
Fig. 2.
HIF-2–dependent up-regulation of CK enzymes. (A) qPCR analysis of CK enzyme (CKM, CKB, CKMT1A) and Cr transporter (SLC6A8) expression in Caco-2 IECs (20% or 1% O2, 6 h; n = 4). (B) Immunoblot analysis of CK levels in Caco-2 IECs (1% O2). (C) Immunoblot analysis of nuclear (HIF) and whole-cell lysates (CK) from Caco-2 treated with vehicle (DMSO) or PHD inhibitor (AKB; 100 μM) for 24 h. (D) qPCR analysis of CK expression in shCtrl, shHIF-1α, or shHIF-2α Caco-2 (20% or 1% O2, 6 h; n = 4) *P < 0.05. (E) Immunoblot analysis of CK levels in shCtrl, shHIF-1α, and shHIF-2α IECs (20% or 1% O2, 24 h). (F, Schematic) Proximal promoter of human CKM and CKB; TSS designated +1. T84 cells were nucleofected (24 h) and cultured in 20% or 1% O2 for 24 h. Data represent ratio of promoter-luc:pRen-luc activity (n = 4; *P < 0.05). (G) Caco-2 IECs were cotransfected with pCK-Luc and pcDNA3.1 vector as control (C) or increasing ratios of pcDNA3.1–HIF-1α∆ODD or pcDNA3.1–HIF-2α∆ODD (n = 3; *P < 0.0001). (H and I) Luciferase activity in lysates cotransfected with full-length (WT), truncated pCK-Luc (schematic) (n = 3; *P < 0.0001), or pCK-Luc harboring mutations in distal (HRE2) and proximal (HRE1) sites, and HIF plasmids at a 1:5 ratio (n = 4; *P < 0.05 by ANOVA).