Significance
The electronic structures of two-dimensional materials can be tuned for a variety of applications by guest species intercalation into the van der Waals gaps. Using Li electrochemical intercalated MoS2 as an example here, we correlate the continuously tuned electronic structure of lithiated MoS2 with the corresponding enhanced hydrogen evolution reaction activity, and thus construct the electronic structure–catalytic activity relationship. This work offers a unique thinking of tuning the electronic structures of layered materials by guest species intercalation for optimizing different kinds of catalysis on the basis of the strong correlation between the electronic structures and catalytic activities of the catalysts.
Keywords: 2D materials, layer vertically standing, electrochemical catalysis
Abstract
The ability to intercalate guest species into the van der Waals gap of 2D layered materials affords the opportunity to engineer the electronic structures for a variety of applications. Here we demonstrate the continuous tuning of layer vertically aligned MoS2 nanofilms through electrochemical intercalation of Li+ ions. By scanning the Li intercalation potential from high to low, we have gained control of multiple important material properties in a continuous manner, including tuning the oxidation state of Mo, the transition of semiconducting 2H to metallic 1T phase, and expanding the van der Waals gap until exfoliation. Using such nanofilms after different degree of Li intercalation, we show the significant improvement of the hydrogen evolution reaction activity. A strong correlation between such tunable material properties and hydrogen evolution reaction activity is established. This work provides an intriguing and effective approach on tuning electronic structures for optimizing the catalytic activity.
Layer-structured 2D materials are an interesting family of materials with strong covalent bonding within molecular layers and weak van der Waals interaction between layers. Beyond intensively studied graphene-related materials (1–4), there has been recent strong interest in other layered materials whose vertical thickness can be thinned down to less than few nanometers and horizontal width can also be reduced to nanoscale (5–9). The strong interest is driven by their interesting physical and chemical properties (2, 10) and their potential applications in transistors, batteries, topological insulators, thermoelectrics, artificial photosynthesis, and catalysis (4, 11–25).
One of the unique properties of 2D layered materials is their ability to intercalate guest species into their van der Waals gaps, opening up the opportunities to tune the properties of materials. For example, the spacing between the 2D layers could be increased by intercalation such as lithium (Li) intercalated graphite or molybdenum disulfide (MoS2) and copper intercalated bismuth selenide (26–29). The electronic structures of the host lattice, such as the charge density, anisotropic transport, oxidation state, and phase transition, may also be changed by different species intercalation (26, 27).
As one of the most interesting layered materials, MoS2 has been extensively studied in a variety of areas such as electrocatalysis (20–22, 30–36). It is known that there is a strong correlation between the electronic structure and catalytic activity of the catalysts (20, 37–41). It is intriguing to continuously tune the morphology and electronic structure of MoS2 and explore the effects on MoS2 hydrogen evolution reaction (HER) activity. Very recent studies demonstrated that the monolayered MoS2 and WS2 nanosheets with 1T metallic phase synthesized by chemical exfoliation exhibited superior HER catalytic activity to those with 2H semiconducting phase (35, 42), with a possible explanation that the strained 1T phase facilitates the hydrogen binding process during HER (42). However, it only offers two end states of materials and does not offer a continuous tuning. A systematic investigation to correlate the gradually tuned electronic structure, including oxidation state shift and semiconducting–metallic phase transition, and the corresponding HER activity is important but unexplored. We believe that the Li electrochemical intercalation method offers a unique way to tune the catalysts for optimization.
In this paper, we demonstrate that the layer spacing, oxidation state, and the ratio of 2H semiconducting to 1T metallic phase of MoS2 HER catalysts were continuously tuned by Li intercalation to different voltages vs. Li+/Li in nanofilms with molecular layers perpendicular to the substrates. Correspondingly, the catalytic activity for HER was observed to be continuously tuned. The lower oxidation state of Mo and 1T metallic phase of MoS2 turn out to have better HER catalytic activities. The performance of MoS2 catalyst on both flat and 3D electrodes was dramatically improved when it was discharged to low potentials vs. Li+/Li.
Results
Synthesis and Characterization.
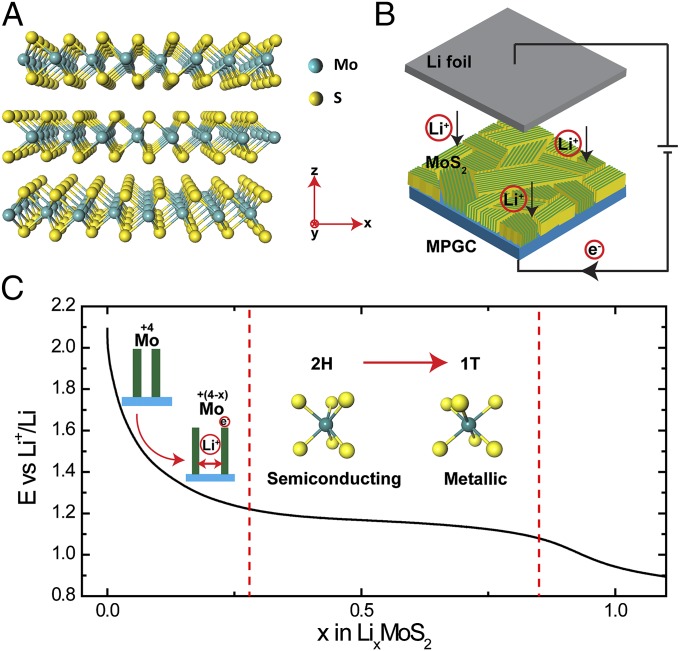
The atomic structure of 2H MoS2 is illustrated in Fig. 1A. Charge neutral molecular layers consisting of covalently bonded Mo and S atoms stack along the z direction by weak van der Waals interaction. To largely expose the active edge sites for HER (20, 21, 43), MoS2 nanofilms on mirror polished glassy carbon (MPGC) with molecular layers perpendicular to the substrate were converted from 12-nm Mo film by a rapid sulfurization process presented in our previous work (43, 44) (Materials and Methods), as illustrated by the schematic in Fig. 1B. The as-grown MoS2 nanofilm together with a piece of Li foil were then made into a pouch battery cell to perform Li electrochemical intercalation through a galvanostatic discharge process and form LixMoS2 compound (Materials and Methods). The battery testing system enabled us to monitor and control the lithiation process at room temperature within only several hours, by which the amount of intercalated Li was continuously tuned as shown in Fig. 1C (29, 45). The discharge curve in Fig. 1C offers us useful information about the tuning of electronic structure of MoS2 during Li intercalation. The voltage of LixMoS2 vs. Li+/Li is decreased monotonically from 2.1 to 1.2 V, during which Li intercalates into MoS2 with the content x increasing to around 0.28 (29, 46). The following lithiation process undergoes a 2H to 1T MoS2 first-order phase transition, as a well-defined discharge plateau is observed between 1.2 and 1.1 V in Fig. 1C (29, 45–47). The 2H and 1T MoS2 have different atomic and electronic structures illustrated in Fig. 1C. In 2H MoS2, which is semiconducting, the Mo atom is coordinated by six S atoms in a trigonal prismatic arrangement, whereas the Mo atom in the 1T phase presents an octahedral S coordination which results in the metallic property (29, 35, 42, 47, 48). Despite the electronic structure change, because the Li between the layers is expected to expand the layer spacing as indicated in Fig. 1C, the morphology of the as-grown MoS2 nanofilm can also be tuned by the lithiation process (29, 45).
Fig. 1.
Schematics and galvanostatic discharge curve of Li electrochemical intercalation into MoS2 nanofilms. (A) Crystal structure of 2H MoS2. (B) Schematic of the battery testing system. The cathode is MoS2 nanofilm with molecular layers perpendicular to the substrate, where the green and yellow colors represent the edge sites and the terrace sites, respectively. The anode is the Li foil. (C) Galvanostatic discharge curve representing the lithiation process. Li intercalates into the van der Waals gaps of MoS2 to donate electrons to the slabs and expand the layer spacing. The voltage monotonically drops to 1.2 V vs. Li+/Li to reach a Li content of 0.28, after which the system undergoes a 2H to 1T MoS2 first-order phase transition. The atomic structure is changed from trigonal prismatic to octahedral, along with the electronic semiconducting to metallic transition.
Characterizations of MoS2 nanofilms stopped at different lithiation voltages were performed after the surface contaminations were removed by ethanol treatment. Different from the recent study of completely exfoliated WS2 monolayers in which Li was not detected (42), electron energy loss spectroscopy (Fig. S1) and inductively coupled plasma mass spectroscopy (ICP-MS) (Materials and Methods; Table S1) results suggest that only part of the intercalated Li in our MoS2 layers vertically aligned structure reacts with ethanol. The tuning of MoS2 layer spacing by Li electrochemical intercalation is confirmed by the transmission electron microscopy (TEM) images of MoS2 discharged to different voltages in Fig. 2. Pristine MoS2 nanofilm on MPGC with molecular layers perpendicular to the substrate is shown in Fig. 2A, followed by Fig. 2 B–E representing MoS2 treated by lithiation from the open circuit voltage around 2.1 to 1.8 (x
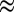 0.02), 1.5 (x
0.02), 1.5 (x
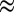 0.07), 1.2 (x
0.07), 1.2 (x
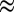 0.28), and 1.1 V (x
0.28), and 1.1 V (x
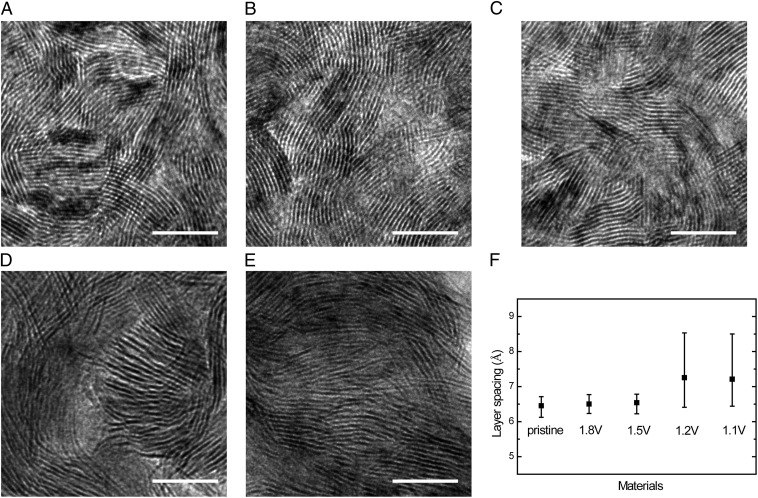
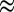 0.85) vs. Li+/Li (Fig. 1C), respectively (these lithiation voltages are used for sampling throughout the entire paper). The films are polycrystalline with in-plane randomly oriented grains around 10 nm in size. As shown in our previous study, the special texture of vertically aligned MoS2 maximally exposes the active edge sites on a 2D surface for HER and reduces the impedance of the charge transfer from the substrate to the surface sites, and at the same time fully opens van der Waals gaps for Li to be intercalated (43, 44). The morphologies of MoS2 at potentials above ∼1.5 V (x < 0.1) (TEM images in Fig. 2 A–C) have little change from Li intercalation. In comparison, some of the layers are obviously expanded and partially exfoliated at potentials below ∼1.2 V (x > 0.28) (Fig. 2 D and E).
0.85) vs. Li+/Li (Fig. 1C), respectively (these lithiation voltages are used for sampling throughout the entire paper). The films are polycrystalline with in-plane randomly oriented grains around 10 nm in size. As shown in our previous study, the special texture of vertically aligned MoS2 maximally exposes the active edge sites on a 2D surface for HER and reduces the impedance of the charge transfer from the substrate to the surface sites, and at the same time fully opens van der Waals gaps for Li to be intercalated (43, 44). The morphologies of MoS2 at potentials above ∼1.5 V (x < 0.1) (TEM images in Fig. 2 A–C) have little change from Li intercalation. In comparison, some of the layers are obviously expanded and partially exfoliated at potentials below ∼1.2 V (x > 0.28) (Fig. 2 D and E).
Fig. 2.
TEM images of MoS2 after Li intercalation. (A) TEM image of pristine MoS2 with molecular layers perpendicular to the substrate. (B–E) TEM images of MoS2 by Li electrochemical intercalation to 1.8, 1.5, 1.2, and 1.1 V vs. Li+/Li, respectively. (F) Averaged layer spacing of pristine and lithiated MoS2. Error bars represent the layer spacing variation of different grains measured in TEM images. Scale bar, 10 nm.
The layer spacing of MoS2 nanofilms lithiated at different voltages is determined by averaging the layer spacing of randomly selected 15 grains in the TEM images (Fig. 2F). Pristine MoS2 layer spacing is measured to be 6.45 Å, consistent with the previous study (49). The spacing of 1.8- and 1.5-V lithiation-treated MoS2 is increased very little to 6.50 and 6.54 Å but still within the range of the error bar, indicating also very little expansion of van der Waals gap. Samples of 1.2 and 1.1 V are dramatically expanded to 7.25 and 7.21 Å, respectively, with increased layer spacing distribution. No other guest species observed in these TEM images rules out any organic intercalation (29, 50). Part of the intercalated Li reacts with ethanol and partially exfoliates MoS2 layers, which is also responsible for the large layer spacing expansion (35, 42, 45, 51). Complete exfoliation happens with observed single layers when MoS2 is treated by a deeper lithiation process (0.8 V) as illustrated in Fig. S2A. Note that the grains in 1.2- and 1.1-V lithiation-treated MoS2 are expanded but not yet completely exfoliated or damaged after the lithiation process, and the morphology of vertically standing molecular layers is still maintained to a certain degree. Scanning electron microscopy (SEM) images of pristine and 1.1-V lithiation-treated MoS2 shown in Fig. S3 also suggest that the film after lithiation treatment still maintains contact with the substrate except for partial detachment originated from the pinholes of the pristine film.
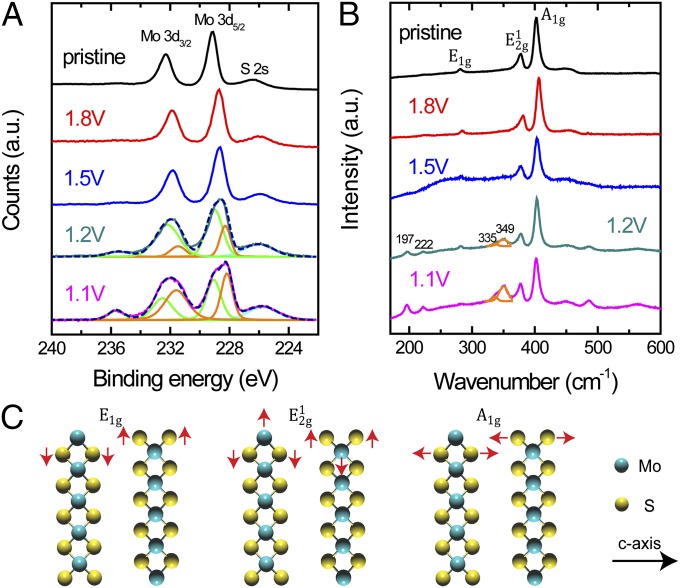
The Li electrochemical intercalation into van der Waals gap has at least three effects on the electronic structure of MoS2. First, the layer spacing is increased after the lithiation and lithium–ethanol reaction processes, which changes the van der Waals interaction between adjacent layers and thus the electronic band structure (45, 52). Second, due to its highly reductive nature, the intercalated Li remaining from reaction with ethanol donates electrons to the MoS2 slabs, which changes the d-band filling and oxidation state of Mo in MoS2 as described by the schematic in Fig. 1C (29, 47, 48, 53, 54). Third, when the content of intercalated Li exceeds 0.28, the 2H semiconducting to 1T metallic MoS2 phase transition happens. Both the oxidation state shift and phase transition of MoS2 are observed in x-ray photoelectron spectroscopy (XPS) spectra in Fig. 3A. All of the spectra were calibrated by a carbon 1s peak located at 284.50 eV (55). Pristine MoS2 shows a Mo 3d5/2 peak at 229.1 eV, indicating a +4 oxidation state of Mo (31). Before the 2H to 1T phase transition happens, Mo 3d5/2 binding energy is continuously shifted to 228.7 and 228.6 eV when MoS2 is discharged to 1.8 and 1.5 V vs. Li+/Li, respectively. The lower binding energies confirm our assumption that the intercalated Li donate electrons to MoS2, resulting in lower oxidation states of Mo (53, 54). Additional peaks start to appear when the 2H to 1T MoS2 phase transition occurs through 1.2 to 1.1 V vs. Li+/Li. Deconvolution of the broad Mo 3d5/2 and Mo 3d3/2 peaks in both spectra presents two independent Mo 3d regions, with a separation of binding energy around 0.8 eV (42, 51). The positions of deconvoluted Mo 3d5/2 peaks in both spectra are located at 229.0 and 228.2 eV, representing the 2H and 1T MoS2 oxidation states, respectively. The composition of the 1T phase in MoS2 nanofilm discharged to 1.1 V vs. Li+/Li is increased compared with the one in the 1.2-V sample, which is indicated by the decreased ratio of 2H to 1T Mo 3d5/2 peak areas from 1.2 (1.53) to 1.1 V (0.86) shown in Fig. 3A (42, 51). Mo 3d5/2 peaks at 235.6 eV with +6 oxidation state are observed in 1.2- and 1.1-V lithiated MoS2, suggesting the slight oxidation during the sample preparation. Fig. S4 shows that the oxidation states of S are maintained at −2 and not affected by Li intercalation when MoS2 is discharged to 1.8 and 1.5 V. Additional peaks of S 2p region showing up at 1.2- and 1.1-V discharging voltage in Fig. S4 again confirm the 1T MoS2 phase formation.
Fig. 3.
Characterizations of pristine and lithiated MoS2 nanofilms. (A) XPS spectra of pristine and Li electrochemically intercalated MoS2. Mo 3d region is shifted toward lower binding energy after Li intercalation to 1.8 and 1.5 V vs. Li+/Li. Mo 3d3/2 and 3d5/2 peaks are then split into two independent regions at 1.2 and 1.1 V vs. Li+/Li, representing the 2H (green line) to 1T (orange line) MoS2 phase transition. (B) Raman spectra of pristine and Li electrochemically intercalated MoS2. Four additional peaks located at 197, 222, 335, and 349 cm−1 are observed in 1.2- and 1.1-V lithiated MoS2, confirming the 1T phase MoS2 formation. The broad peak left of the E12g peak is deconvoluted into two peaks. (C) Atomic vibration direction of E1g, E12g, and A1g Raman modes of MoS2.
Raman spectra in Fig. 3B offer us more information about the morphology and phase transition of pristine and lithiated MoS2. Fig. 3C illustrates the E1g, E12g, and A1g vibration modes located at 281, 377, and 403 cm−1, respectively, in pristine MoS2 on MPGC with the calibration of Si peak at 520.0 cm−1 (ref. 56). The integrated intensity of A1g is nearly three times that of E12g, which suggests the texture of as-grown MoS2 with molecular layers vertically standing on the substrate that favors the vibration of A1g mode (43, 44). The 1.8- and 1.5-V lithiation-treated MoS2 have nearly the same spectra with the pristine one, showing no phase or structure changes. As the content of intercalated Li is increased, more and more electrons are donated from Li to MoS2 slabs, which results in the 2H to 1T phase transition to lower the LixMoS2 electronic energy (29, 48, 53). Four additional peaks, located, respectively, at 197, 222, 335, and 349 cm−1, indicate the 2H to 1T MoS2 phase transition when MoS2 was discharged to 1.2 and 1.1 V vs. Li+/Li (35, 47, 48, 51). The increased intensities of the emerged peaks further confirm that 1.1-V discharged MoS2 has more 1T phase than that in the 1.2-V sample.
Electrochemical Testing.
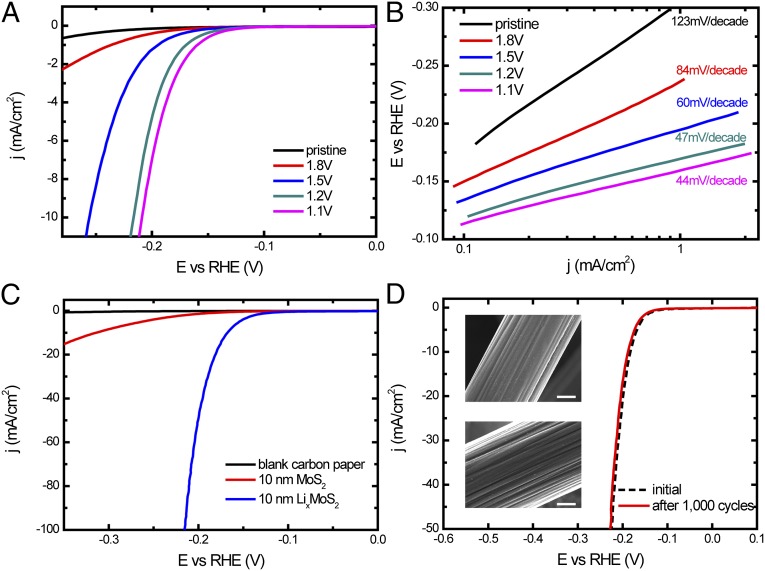
Correlating the tunable layer spacing and electronic structure of MoS2 by Li electrochemical intercalation and the corresponding HER catalytic activities offers clear insight (35, 42). The electrochemical testing of pristine and lithiated MoS2 with mass loading around 22 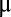 g/cm2 on MPGC were performed in 0.5 M H2SO4 solution with a typical three-electrode electrochemical cell setup (Materials and Methods). Before the polarization curve was tested the sample was cycled several times by taking continuous cyclic voltammograms (CVs) between −0.25 and 0.1 V vs. reversible hydrogen electrode (RHE) before iR correction to remove contaminants on the surface. Electrochemical impedance spectroscopy reveals negligible ohmic resistance of the films (Fig. S5). All of the results have been iR-corrected by subtracting the ohmic resistance loss from the overpotential. It turns out that MoS2 HER activity can be continuously tuned by Li electrochemical intercalation to different voltages vs. Li+/Li, as shown in Fig. 4 A and B. The pristine MoS2 exhibits a Tafel slope of 123 mV per decade and an exchange current density of 3.4
g/cm2 on MPGC were performed in 0.5 M H2SO4 solution with a typical three-electrode electrochemical cell setup (Materials and Methods). Before the polarization curve was tested the sample was cycled several times by taking continuous cyclic voltammograms (CVs) between −0.25 and 0.1 V vs. reversible hydrogen electrode (RHE) before iR correction to remove contaminants on the surface. Electrochemical impedance spectroscopy reveals negligible ohmic resistance of the films (Fig. S5). All of the results have been iR-corrected by subtracting the ohmic resistance loss from the overpotential. It turns out that MoS2 HER activity can be continuously tuned by Li electrochemical intercalation to different voltages vs. Li+/Li, as shown in Fig. 4 A and B. The pristine MoS2 exhibits a Tafel slope of 123 mV per decade and an exchange current density of 3.4  10−3 mA/cm2, showing comparably low HER catalytic activity (43). However, the Tafel slope is reduced to 84 and 60 mV per decade after MoS2 is discharged to 1.8 and 1.5 V vs. Li+/Li, respectively, before the 2H to 1T phase transition. The HER catalytic activity is then further improved by Li electrochemical intercalation to 1.2 and 1.1 V and saturated to a Tafel slope around 44 mV per decade, which agrees well with the exfoliated MoS2 nanosheets by n-butyl Li solution (35). The 1.1-V lithiated MoS2 has a slightly improved HER activity than that of 1.2 V. Deeper Li discharge process, for example 0.8 V vs. Li+/Li lithiation, may completely exfoliate MoS2 layers and destroy the bonds between the active material and the substrate upon exposure to aqueous electrolyte which performs degraded activity (Fig. S2B) (35, 45). Some previous studies suggested the reduction of MoS2 to Mo metal and Li2S when it was discharged to low potentials, which is another possibility to explain the activity degradation (45). Several identical samples at each lithiation voltages were tested to ensure the reproducibility of the results, as summarized in Table 1.
10−3 mA/cm2, showing comparably low HER catalytic activity (43). However, the Tafel slope is reduced to 84 and 60 mV per decade after MoS2 is discharged to 1.8 and 1.5 V vs. Li+/Li, respectively, before the 2H to 1T phase transition. The HER catalytic activity is then further improved by Li electrochemical intercalation to 1.2 and 1.1 V and saturated to a Tafel slope around 44 mV per decade, which agrees well with the exfoliated MoS2 nanosheets by n-butyl Li solution (35). The 1.1-V lithiated MoS2 has a slightly improved HER activity than that of 1.2 V. Deeper Li discharge process, for example 0.8 V vs. Li+/Li lithiation, may completely exfoliate MoS2 layers and destroy the bonds between the active material and the substrate upon exposure to aqueous electrolyte which performs degraded activity (Fig. S2B) (35, 45). Some previous studies suggested the reduction of MoS2 to Mo metal and Li2S when it was discharged to low potentials, which is another possibility to explain the activity degradation (45). Several identical samples at each lithiation voltages were tested to ensure the reproducibility of the results, as summarized in Table 1.
Fig. 4.
Electrochemical characterization of pristine and lithiated MoS2 on MPGC and CFP. (A) Polarization curves of pristine and lithiated MoS2 on MPGC, with the catalytic activities improved by lower voltages Li intercalation. (B) Tafel slope of MoS2 is continuously tuned by Li electrochemical intercalation to different voltages. The slope reaches around 44 mV per decade at 1.1 V vs. Li+/Li. (C) HER activity of ALD MoS2 on CFP is dramatically improved by Li intercalation. The Tafel slope is enhanced from 98 mV per decade (pristine MoS2) to 44 mV per decade (lithiated MoS2). (D) Electrochemical stability test of lithiated ALD MoS2. The catalytic activity shows no obvious degradation after the cycling. (Insets) SEM images of the catalyst before (Upper) and after (Lower) 1,000 cycles showing no obvious change. Scale bar, 2 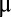 m.
m.
Table 1.
Electrochemical analysis of pristine and lithiated MoS2 on MPGC
| Materials | Tafel slope (mV per decade) | Exchange current density, 10−3 mA/cm2 | η (mV) @ j = 0.1 mA/cm2 | j (mA/cm2) @ η = 200 mV |
| Pristine MoS2 | 115∼123 | 1.71∼3.40 | −173 | −0.15 |
| 1.8 V MoS2 | 75∼86 | 0.80∼1.62 | −150 | −0.40 |
| 1.5 V MoS2 | 56∼73 | 0.56∼0.93 | −133 | −1.21 |
| 1.2 V MoS2 | 46∼51 | 0.10∼0.38 | −119 | −4.96 |
| 1.1 V MoS2 | 43∼47 | 0.13∼0.25 | −113 | −6.74 |
Tafel slopes and exchange current densities were obtained by several identical samples, and the overpotentials and cathodic current densities were obtained from Fig. 4A.
A high-surface-area 3D carbon fiber paper (CFP) was used to improve the overall HER performance of Li electrochemically intercalated MoS2. Atomic layer deposition (ALD) is an established technique to deliver materials to high-aspect-ratio nanostructures in a conformal and ultrathin fashion (57–59). We conformally coated CFP with MoO3 by ALD (Materials and Methods) (60), which was then converted to MoS2 by the same rapid sulfurization process (the as-grown catalyst is donated as ALD MoS2). The synthesis of ALD MoS2 with molecular layers perpendicular to the substrate was confirmed by the TEM image and Raman spectrum shown in Fig. S6 A and B. Benefitting from the maximally exposed active edge sites, the thickness of MoS2 nanofilm can be lowered down to reduce the loading of the catalyst while maintaining the high HER catalytic activity. Ten nm MoO3 was deposited onto CFP and then converted to 10 nm MoS2 with a mass loading around 120 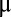 g/cm2. The HER catalytic activity of pristine ALD MoS2 on CFP is shown in Fig. 4A with a Tafel slope of 98 mV per decade and exchange current density of 100
g/cm2. The HER catalytic activity of pristine ALD MoS2 on CFP is shown in Fig. 4A with a Tafel slope of 98 mV per decade and exchange current density of 100  10−3 mA/cm2 (Fig. S6C). The Tafel slope is dramatically improved to 44 mV per decade after discharging to 0.7 V vs. Li+/Li, achieving much higher HER catalytic activity as shown in Fig. 4C and Fig. S6C (due to the different substrate and precursor used in the synthesis, the 1.2- and 1.1-V discharged ALD MoS2 on CFP did not exhibit the best activity). Only 168- and 216-mV overpotentials (
10−3 mA/cm2 (Fig. S6C). The Tafel slope is dramatically improved to 44 mV per decade after discharging to 0.7 V vs. Li+/Li, achieving much higher HER catalytic activity as shown in Fig. 4C and Fig. S6C (due to the different substrate and precursor used in the synthesis, the 1.2- and 1.1-V discharged ALD MoS2 on CFP did not exhibit the best activity). Only 168- and 216-mV overpotentials (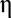 ) are needed for lithiated ALD MoS2 to drive 10- and 100-mA/cm2 cathodic current density (j), respectively. Electrochemical stability of the catalyst is of our concern as the Li intercalation process could introduce structure distortion and layer exfoliation, which can weaken the bond between ALD MoS2 film and CFP substrate. The stability of lithiated ALD MoS2 was tested by taking 1,000 continuous CV cycles between −0.19 and 0.1 V vs. RHE to reach 10 mA/cm2 without iR correction. Fig. 4D shows that after 1,000 cycles the catalyst maintains high HER activity with negligible degradation. The SEM images of the catalyst before and after the cycling indicate that the film is intact. After the cycling test, 8.6% of Li was still maintained in the MoS2 matrix (Table S1). In addition, stable electrocatalytic currents around 10 and 20 mA/cm2 were observed for at least 5 h when constant voltages at 175 and 190 mV were applied to the catalyst, respectively (Fig. S6D). The MoS2 molecular layers vertically standing on the substrate can create strong bonds to the surface, which is assumed to explain the good stability even after Li intercalation (44). In addition, the good stability demonstrates that 1T phase MoS2 is maintained during the hydrogen evolution process (35, 42, 51).
) are needed for lithiated ALD MoS2 to drive 10- and 100-mA/cm2 cathodic current density (j), respectively. Electrochemical stability of the catalyst is of our concern as the Li intercalation process could introduce structure distortion and layer exfoliation, which can weaken the bond between ALD MoS2 film and CFP substrate. The stability of lithiated ALD MoS2 was tested by taking 1,000 continuous CV cycles between −0.19 and 0.1 V vs. RHE to reach 10 mA/cm2 without iR correction. Fig. 4D shows that after 1,000 cycles the catalyst maintains high HER activity with negligible degradation. The SEM images of the catalyst before and after the cycling indicate that the film is intact. After the cycling test, 8.6% of Li was still maintained in the MoS2 matrix (Table S1). In addition, stable electrocatalytic currents around 10 and 20 mA/cm2 were observed for at least 5 h when constant voltages at 175 and 190 mV were applied to the catalyst, respectively (Fig. S6D). The MoS2 molecular layers vertically standing on the substrate can create strong bonds to the surface, which is assumed to explain the good stability even after Li intercalation (44). In addition, the good stability demonstrates that 1T phase MoS2 is maintained during the hydrogen evolution process (35, 42, 51).
Discussion
It is always important to construct the electronic structure–catalytic activity relationship in electrocatalysis (20, 37–41). As the H binding energy and activation barrier on the active edge sites are strongly related to the electronic structure of MoS2 (20, 37), it is effective to continuously tune and improve the HER catalytic activity by continuously tuning the electronic structure, especially the oxidation state of Mo and 2H to 1T phase transition, through Li electrochemical intercalation. We find that even before the 2H to 1T phase transition occurs, HER activities are already improved along with the lowered oxidation states of Mo. The lowered oxidation states of Mo might have influence on the electron filling of bonding and antibonding between the active sites and H, which significantly changes the H bonding energy and activation barrier and thus improves the HER activity. Different from the recent studies in which MoS2 and WS2 are totally exfoliated to be monolayered nanosheets (35, 42), the morphology of MoS2 in this study is not destroyed by Li intercalation and Li reaction with ethanol or water. Thus, we suspect that there is still Li trapped between the layers to anchor the oxidation state of Mo during the electrochemical test in which some of the intercalated Li could react with water to produce Li(OH) and H2 (35, 42, 45, 51). Suggested by the improved HER activity of 1.1-V lithiated MoS2 from 1.2 V, we also conclude that 1T MoS2 shows superior HER activity to the 2H phase, which agrees with some recent results (35, 42). The XPS spectrum of 1.1-V lithiated MoS2 before and after electrochemical cycling in Fig. S7 shows no obvious change in the 1T to 2H phase ratio, indicating that the electronic structure is maintained during hydrogen generation process (35, 42, 51). MoS2 electronic structure changed by expanding the layers through Li intercalation and partial exfoliation, as shown in Fig. 2, could also be a possible explanation for the tunable HER activity (52, 54). In addition, Li intercalation improves the conductivity of MoS2, which is also important to the improved HER activity of lithiated MoS2.
In conclusion, we synthesized MoS2 nanofilm with molecular layers perpendicular to the substrates. By Li electrochemical intercalation to different voltages vs. Li+/Li, the layer spacing and electronic structure of MoS2 was continuously tuned, thus tuning the HER catalytic activity effectively.
Materials and Methods
Material Synthesis and Li Intercalation.
Molybdenum oxide layers were deposited by ALD on CFP (Fuel Cell Store) in a customized ALD–ozone system. Molybdenum hexacarbonyl (99.9%, Sigma-Aldrich) was used as the molybdenum precursor. Ozone was generated from a mixture of oxygen (99.99%) and nitrogen (99.998%, with mixing concentration of 50 ppm) in an ozone generator (MKS, AX8407-C2). The feeding oxygen flow rate was kept at ∼500 standard cubic centimeters per minute (sccm), providing an ozone concentration of 21.7 wt %. The ozone flow into the chamber was controlled by a mass flow controller at 200 sccm. The substrate temperature was kept at 165 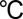 , the molybdenum precursor at 60
, the molybdenum precursor at 60 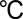 , and ozone feed line at 120
, and ozone feed line at 120 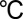 . An exposure mode was adopted for deposition on carbon papers. The molybdenum precursor were exposed for 0.5 s and soaked in the chamber for another 1 s via a stop valve before argon (99.999%) purging. The growth rates and thickness of the films were calibrated by 10 cm
. An exposure mode was adopted for deposition on carbon papers. The molybdenum precursor were exposed for 0.5 s and soaked in the chamber for another 1 s via a stop valve before argon (99.999%) purging. The growth rates and thickness of the films were calibrated by 10 cm  10 mm witness silicon pieces (P-type test wafer 0–100 Ω-cm 500 µm single-sided polished, University Wafer). For ALD on CFP, weight changes were also tracked as a function of ALD cycle numbers to provide complementary information about the growth rate. From the witness wafer piece, the coated CFP in this article was expected to have an average film thickness of 100
10 mm witness silicon pieces (P-type test wafer 0–100 Ω-cm 500 µm single-sided polished, University Wafer). For ALD on CFP, weight changes were also tracked as a function of ALD cycle numbers to provide complementary information about the growth rate. From the witness wafer piece, the coated CFP in this article was expected to have an average film thickness of 100  5. Å The surface area of CFP was calculated to be 29 cm2/cm2. To verify conformal coating, Auger element mapping was conducted on the molybdenum-oxide-coated CFP shown in Fig. S8. By comparing the SEM images before and after ALD as shown by Fig. S8 A and B, there were no drastic morphology changes during the deposition process. The element mapping shown in Fig. S8 C–E confirmed that Mo and O are uniformly distributed.
5. Å The surface area of CFP was calculated to be 29 cm2/cm2. To verify conformal coating, Auger element mapping was conducted on the molybdenum-oxide-coated CFP shown in Fig. S8. By comparing the SEM images before and after ALD as shown by Fig. S8 A and B, there were no drastic morphology changes during the deposition process. The element mapping shown in Fig. S8 C–E confirmed that Mo and O are uniformly distributed.
Edge-terminated MoS2 nanofilms on MPGC (HTW Hochtemperatur Werkstoffe Gmbh) and CFP were grown inside a single-zone, 12-inch horizontal tube furnace (Lindberg/Blue M) equipped with a 1-inch-diameter quartz tube. The substrates coated with Mo or MoO3 thin films were placed at the hot center of the tube furnace. Sulfur powder (99.99%, Sigma Aldrich) was placed on the upstream side of the furnace at carefully adjusted locations to set the temperature. Ar gas was used as the precursor carrier and the pressure and flow rate were kept at 1,000 mtorr and 100 sccm, respectively, during the growth. The heating center of the furnace was quickly raised to reaction temperature of 600 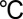 in 20 min, and the sulfur precursor was kept at around 200
in 20 min, and the sulfur precursor was kept at around 200 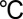 . The furnace was held at reaction temperature for 10 min, followed by natural cool-down.
. The furnace was held at reaction temperature for 10 min, followed by natural cool-down.
The as-grown MoS2 was then made into a battery configuration acting as the cathode, combined with Li metal as anode and 1.0 M LiPF6 in 1:1 wt/wt ethylene carbonate/diethyl carbonate (EMD Chemicals) as electrolyte. The galvanostatic discharge currents for MoS2 samples were set at 200 mA/g. After the discharge process, samples were cleaned by ethanol to remove the electrolyte on the surface. ICP-MS samples were prepared in 5% HNO3 solution by taking continuous CV cycles between 0 and 1.2 V vs. a saturated calomel electrode (SCE) to oxidize and dissolve Li and Mo into the solution.
Characterizations.
Characterizations were carried out using TEM (FEI Tecnai G2 F20 X-Twin microscope at 200 keV), Raman spectroscopy (531-nm excitation laser, cutoff around 175 cm−1, WITEC Raman spectrometer), XPS [SSI SProbe XPS spectrometer with Al(Ka) source], SEM (FEI Nova NanoSEM 450), and Auger electron spectroscopy (PHI 700 Scanning Auger Nanoprobe).
Electrochemical Measurements.
MoS2 nanofilms on MPGC and CFP were tested in 0.5 M H2SO4 solution (deaerated by N2) using a typical three-electrode electrochemical cell setup, with an SCE [E(RHE) = E(SCE) + 0.280 V after calibration] as the reference electrode and a graphite rod (99.999%, Sigma-Aldrich) as the counter electrode. Electrochemically inert wax (Apiezon wax W-W100) and kapton tape were used to define the 1-cm2 electrode area. Linear sweep voltammetry (scan rate 2 mV/s), CV (scan rate 100 mV/s), and ac impedance spectroscopy (at zero overpotential) were recorded by a Biologic VSP potentiostat.
Supplementary Material
Acknowledgments
We acknowledge support by the Department of Energy, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under Contract DE-AC02-76-SFO0515. This work was also supported as part of the Center on Nanostructuring for Efficient Energy Conversion at Stanford University, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award DE-SC0001060.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316792110/-/DCSupplemental.
References
- 1.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Tan Y-W, Stormer HL, Kim P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature. 2005;438(7065):201–204. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 3.Stankovich S, et al. Graphene-based composite materials. Nature. 2006;442(7100):282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Wang X, Zhang L, Lee S, Dai H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science. 2008;319(5867):1229–1232. doi: 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- 5.Novoselov KS, et al. Two-dimensional atomic crystals. Proc Natl Acad Sci USA. 2005;102(30):10451–10453. doi: 10.1073/pnas.0502848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koski KJ, Cui Y. The new skinny in two-dimensional nanomaterials. ACS Nano. 2013;7(5):3739–3743. doi: 10.1021/nn4022422. [DOI] [PubMed] [Google Scholar]
- 7.Butler SZ, et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano. 2013;7(4):2898–2926. doi: 10.1021/nn400280c. [DOI] [PubMed] [Google Scholar]
- 8.Coleman JN, et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science. 2011;331(6017):568–571. doi: 10.1126/science.1194975. [DOI] [PubMed] [Google Scholar]
- 9.Tenne R. Advances in the synthesis of inorganic nanotubes and fullerene-like nanoparticles. Angew Chem Int Ed Engl. 2003;42(42):5124–5132. doi: 10.1002/anie.200301651. [DOI] [PubMed] [Google Scholar]
- 10.Geim AK. Graphene: Status and prospects. Science. 2009;324(5934):1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 11.Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A. Single-layer MoS2 transistors. Nat Nanotechnol. 2011;6(3):147–150. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- 12.Zhan Y, Liu Z, Najmaei S, Ajayan PM, Lou J. Large-area vapor-phase growth and characterization of MoS(2) atomic layers on a SiO(2) substrate. Small. 2012;8(7):966–971. doi: 10.1002/smll.201102654. [DOI] [PubMed] [Google Scholar]
- 13.Mizushima K, Jones PC, Wiseman PJ, Goodenough JB. LixCoO2 (0<x<-1): A new cathode material for batteries of high energy density. Mater Res Bull. 1980;15(6):783–789. [Google Scholar]
- 14.Shu ZX, McMillan RS, Murray JJ. Electrochemical intercalation of lithium into graphite. J Electrochem Soc. 1993;140(4):922–927. [Google Scholar]
- 15.Zhang H, et al. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat Phys. 2009;5(6):438–442. [Google Scholar]
- 16.Peng H, et al. Aharonov-Bohm interference in topological insulator nanoribbons. Nat Mater. 2010;9(3):225–229. doi: 10.1038/nmat2609. [DOI] [PubMed] [Google Scholar]
- 17.Kong D, et al. Topological insulator nanowires and nanoribbons. Nano Lett. 2010;10(1):329–333. doi: 10.1021/nl903663a. [DOI] [PubMed] [Google Scholar]
- 18.Poudel B, et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science. 2008;320(5876):634–638. doi: 10.1126/science.1156446. [DOI] [PubMed] [Google Scholar]
- 19.McKone JR, Pieterick AP, Gray HB, Lewis NS. Hydrogen evolution from Pt/Ru-coated p-type WSe2 photocathodes. J Am Chem Soc. 2013;135(1):223–231. doi: 10.1021/ja308581g. [DOI] [PubMed] [Google Scholar]
- 20.Hinnemann B, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc. 2005;127(15):5308–5309. doi: 10.1021/ja0504690. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo TF, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science. 2007;317(5834):100–102. doi: 10.1126/science.1141483. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, et al. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Mol Ther. 2013;4:1444. doi: 10.1038/ncomms2472. [DOI] [PubMed] [Google Scholar]
- 23.Farrington GC, Briant JL. Fast ionic transport in solids. Science. 1979;204(4400):1371–1379. doi: 10.1126/science.204.4400.1371. [DOI] [PubMed] [Google Scholar]
- 24.Whittingham MS. Chemistry of intercalation compounds: Metal guests in chalcogenide hosts. Prog Solid State Chem. 1978;12(1):41–99. [Google Scholar]
- 25.Whittingham MS. The role of ternary phases in cathode reactions. J Electrochem Soc. 1976;123(3):315–320. [Google Scholar]
- 26.Koski KJ, et al. High-density chemical intercalation of zero-valent copper into Bi2Se3 nanoribbons. J Am Chem Soc. 2012;134(18):7584–7587. doi: 10.1021/ja300368x. [DOI] [PubMed] [Google Scholar]
- 27.Koski KJ, et al. Chemical intercalation of zerovalent metals into 2D layered Bi2Se3 nanoribbons. J Am Chem Soc. 2012;134(33):13773–13779. doi: 10.1021/ja304925t. [DOI] [PubMed] [Google Scholar]
- 28.Yoo E, et al. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008;8(8):2277–2282. doi: 10.1021/nl800957b. [DOI] [PubMed] [Google Scholar]
- 29.Py MA, Haering RR. Structural destabilization induced by lithium intercalation in MoS2 and related compounds. Can J Phys. 1983;61(1):76–84. [Google Scholar]
- 30.Chen Z, et al. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011;11(10):4168–4175. doi: 10.1021/nl2020476. [DOI] [PubMed] [Google Scholar]
- 31.Kibsgaard J, Chen Z, Reinecke BN, Jaramillo TF. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat Mater. 2012;11(11):963–969. doi: 10.1038/nmat3439. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc. 2011;133(19):7296–7299. doi: 10.1021/ja201269b. [DOI] [PubMed] [Google Scholar]
- 33.Merki D, Hu X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ Sci. 2011;4(10):3878–3888. [Google Scholar]
- 34.Vrubel H, Merki D, Hu X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ Sci. 2012;5(3):6136–6144. [Google Scholar]
- 35.Lukowski MA, et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc. 2013;135(28):10274–10277. doi: 10.1021/ja404523s. [DOI] [PubMed] [Google Scholar]
- 36.Karunadasa HI, et al. A molecular MoS₂ edge site mimic for catalytic hydrogen generation. Science. 2012;335(6069):698–702. doi: 10.1126/science.1215868. [DOI] [PubMed] [Google Scholar]
- 37.Bollinger MV, Jacobsen KW, Norskov JK. Atomic and electronic structure of MoS2 nanoparticles. Phys Rev B. 2003;67(8):085410-1–085410-17. [Google Scholar]
- 38.Kanan MW, et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J Am Chem Soc. 2010;132(39):13692–13701. doi: 10.1021/ja1023767. [DOI] [PubMed] [Google Scholar]
- 39.Vojvodic A, Nørskov JK. Chemistry. Optimizing perovskites for the water-splitting reaction. Science. 2011;334(6061):1355–1356. doi: 10.1126/science.1215081. [DOI] [PubMed] [Google Scholar]
- 40.Subbaraman R, et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat Mater. 2012;11(6):550–557. doi: 10.1038/nmat3313. [DOI] [PubMed] [Google Scholar]
- 41.Suntivich J, May KJ, Gasteiger HA, Goodenough JB, Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011;334(6061):1383–1385. doi: 10.1126/science.1212858. [DOI] [PubMed] [Google Scholar]
- 42.Voiry D, et al. Enhanced catalytic activity in strained chemically exfoliated WS₂ nanosheets for hydrogen evolution. Nat Mater. 2013;12(9):850–855. doi: 10.1038/nmat3700. [DOI] [PubMed] [Google Scholar]
- 43.Kong D, et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013;13(3):1341–1347. doi: 10.1021/nl400258t. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, et al. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 2013;13(7):3426–3433. doi: 10.1021/nl401944f. [DOI] [PubMed] [Google Scholar]
- 45.Zeng Z, et al. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew Chem Int Ed Engl. 2011;50(47):11093–11097. doi: 10.1002/anie.201106004. [DOI] [PubMed] [Google Scholar]
- 46.Imanishi N, Toyoda M, Takeda Y, Yamamoto O. Study on lithium intercalation into MoS2. Solid State Ion. 1992;58(3–4):333–338. [Google Scholar]
- 47.Julien CM. Lithium intercalated compounds: Charge transfer and related properties. Mater Sci Eng Rep. 2003;40(2):47–102. [Google Scholar]
- 48.Yang D, Sandoval SJ, Divigalpitiya WMR, Irwin JC, Frindt RF. Structure of single-molecular-layer MoS2. Phys Rev B Condens Matter. 1991;43(14):12053–12056. doi: 10.1103/physrevb.43.12053. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Kuriyama N, Yuan H, Takeshita HT, Sakai T. Electrochemical hydrogen storage in MoS2 nanotubes. J Am Chem Soc. 2001;123(47):11813–11814. doi: 10.1021/ja017121z. [DOI] [PubMed] [Google Scholar]
- 50.Divigalpitiya WM, Frindt RF, Morrison SR. Inclusion systems of organic molecules in restacked single-layer molybdenum disulfide. Science. 1989;246(4928):369–371. doi: 10.1126/science.246.4928.369. [DOI] [PubMed] [Google Scholar]
- 51.Eda G, et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011;11(12):5111–5116. doi: 10.1021/nl201874w. [DOI] [PubMed] [Google Scholar]
- 52.Frey GL, Elani S, Homyonfer M, Feldman Y, Tenne R. Optical-absorption spectra of inorganic fullerenelike MS2 (M=Mo, W) Phys Rev B. 1998;57(11):6666–6671. [Google Scholar]
- 53.Rocquefelte X, et al. Mo cluster formation in the intercalation compound LiMoS2. Phys Rev B. 2000;62(4):2397–2400. [Google Scholar]
- 54.Wu S, et al. Electrochemically reduced single-layer MoS₂ nanosheets: characterization, properties, and sensing applications. Small. 2012;8(14):2264–2270. doi: 10.1002/smll.201200044. [DOI] [PubMed] [Google Scholar]
- 55.Moulder JF, Stickle WF, Sobol PE, Bomben KD. Handbook of X-ray Photoelectron Spectroscopy. Chanhassen, MN: ULVAC-PHI, Inc., Chigasaki, Japan; Physical Electronics USA, Inc; 1995. pp. 40–41. [Google Scholar]
- 56.Li B, Yu D, Zhang S-L. Raman spectral study of silicon nanowires. Phys Rev B. 1999;59(3):1645–1648. [Google Scholar]
- 57.Elam JW, Dasgupta NP, Prinz FB. ALD for clean energy conversion, utilization, and storage. MRS Bull. 2011;36(11):899–906. [Google Scholar]
- 58.Leskela M, Ritala M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films. 2002;409(1):138–146. [Google Scholar]
- 59.Dasgupta NP, Liu C, Andrews S, Prinz FB, Yang P. Atomic layer deposition of platinum catalysts on nanowire surfaces for photoelectrochemical water reduction. J Am Chem Soc. 2013;135(35):12932–12935. doi: 10.1021/ja405680p. [DOI] [PubMed] [Google Scholar]
- 60.Diskus M, Nilsen O, Fjellvag H. Growth of thin films of molybdenum oxide by atomic layer deposition. J Mater Chem. 2011;21(3):705–710. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.