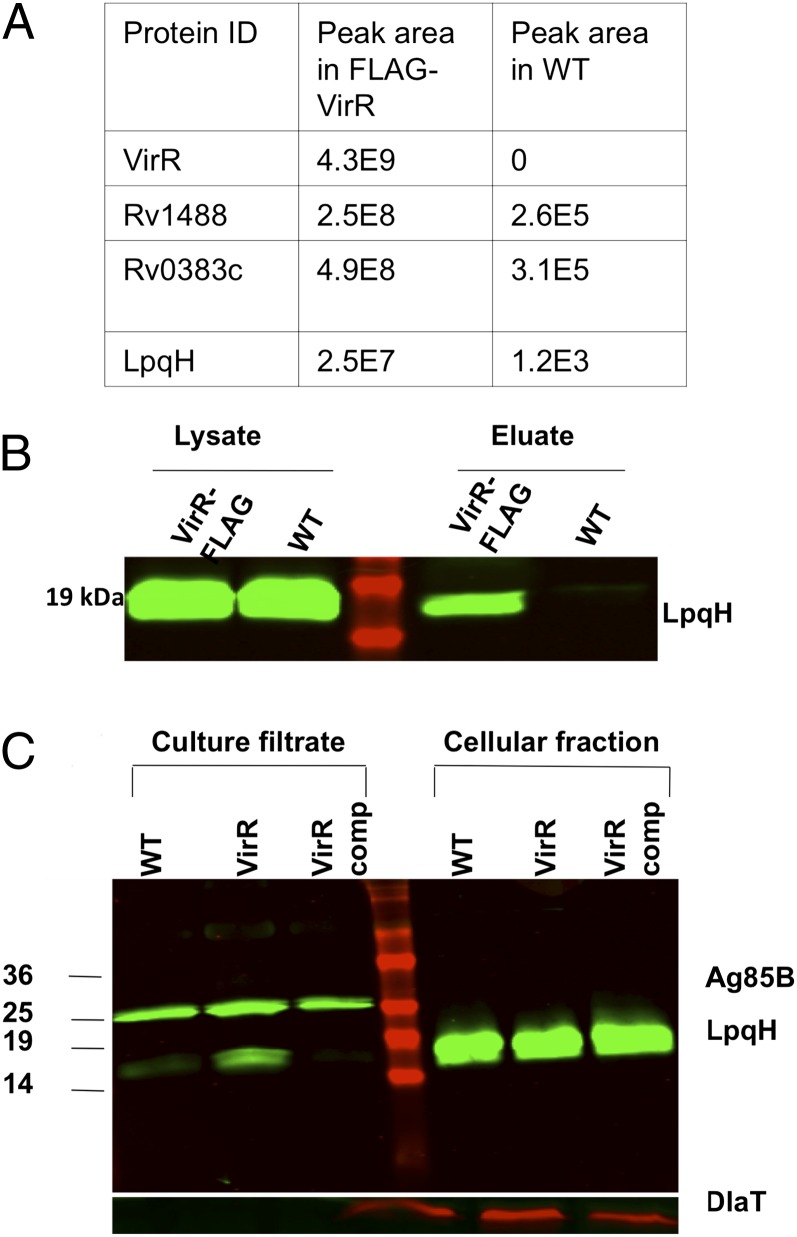
Fig. 4.
VirR is associated with LpqH, and culture filtrate derived from VirR-deficient Mtb is enriched in LpqH. (A) Lysates of FLAG-tagged VirR expressing Mtb and WT Mtb, grown to midlog phase, were immunoprecipitated with anti-FLAG antibody. The eluates were reduced, denatured, and subjected to SDS/PAGE, followed by mass spectrometry for identification of the binding partners. As indicated by the peak areas shown in the table, VirR was pulled down only in the FLAG-Rv0431 lysates, and LpqH was detected to a significantly greater extent in the FLAG-Rv0431 lysates, suggesting that the pulldown was anti-FLAG antibody specific. (B) To confirm binding of LpqH with VirR, native-coimmunoprecipitation was performed. WT and VirR-deficient Mtb lysates were immunoprecipitated with antiserum against VirR, and the eluates were immunoblotted with the same antiserum. The precleared lysates were loaded alongside eluates to confirm equal protein concentration between samples. (C) Culture filtrates and whole-cell lysates derived from WT, VirR-deficient, and VirR-complemented Mtb were immunoblotted with antiserum against LpqH. To ensure equal loading of culture filtrates among strains, samples were immunoblotted with antiserum against Ag85B. To ensure equal loading of lysates among strains and to rule out significant cell lysis, samples were immunoblotted with antisera against DlaT.