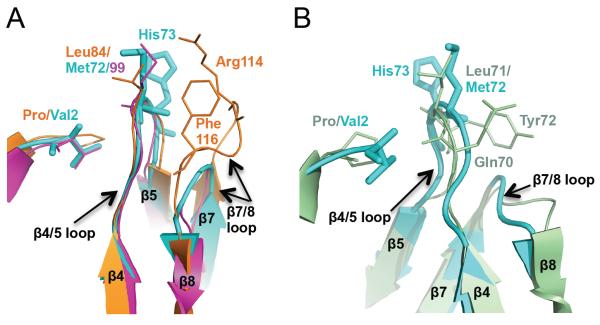
Figure 3.
Void-filling residues in MvNei2, MvNei1 and EcoNei (A) Superposition of the β4/5 loop with the void-filling methionine (Met72 in MvNei2, cyan, and Met99 in MmuNeil3, fuchsia, PDB 3W0F [16]) or leucine (Leu84 in MvNei1, orange, PDB 3A46 [27]) and β7/8 loop with void-filling Arg114 and Phe116 of MvNei1 (orange). (B) Superposition of MvNei2 (cyan) with EcoNei (pale green, PDB 1K3W [20]) showing the similarity in the β4/5 and β7/8 loops in these two enzymes. The β4/5 loops contains the void-filling triad in EcoNei, i.e. Gln70, Leu71, and Tyr72. The β7/8 loops are of similar size and shape, and much shorter than that seen in MvNei1 or hNEIL1. Superpositions were performed with residues 67–78 of MvNei2 and the corresponding residues in MmuNeil3, MvNei1, and EcoNei using a least-squares superposition tool (COOT [41]).