Figure 1.
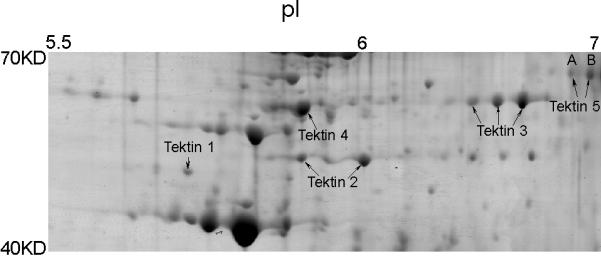
Five tektin members present in flagellar accessory structures. Sperm were processed by 1% S-EDTA, homogenized. Detached head and tail remnants were then separated by sucrose gradient centrifugation. The enriched sperm tail fraction (flagellar accessory structures) was further solubilized in 2D sample buffer. The protein sample (~900 μg) was separated by IEF on a non-linear pH 3–11 IPG strip, followed by a second dimension of SDS-PAGE. The gel was stained with colloidal Coomassie Blue. Gel pieces containing individual protein spots were excised and treated with trypsin. The resulting peptides were analyzed by mass spectrometry for protein identification. All identified tektins are labeled. Based on the two-dimensional gel data, the molecular weights of the two TEKT5 spots (A, B) were around 62,000 Mr, and the isoelectric points were near 7, which corresponded to the respective values of 62,730 and 8.83 predicted by the MacVector computer program. The molecular weights of the tektins were estimated by comparison to the theoretical molecular weights of other proteins identified in this analysis.
