Table 1.
MCR products: synthetic yields, antiproliferative and apoptosis-inducing properties
| Compound |
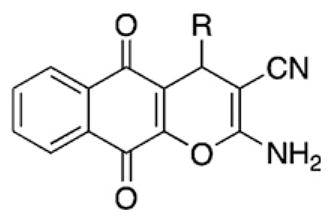 R |
Synthesis % yield | GI50a (μM)
|
% Apoptosisb Jurkat | ||
|---|---|---|---|---|---|---|
| HeLa | MCF-7/AZ | Jurkat | ||||
| 1 | 3-PhO-C6H4- | 82 | 4.0 ± 2.9 | 2.2 ± 0.4 | 17.9 ± 1.8 | 50 ± 1.9 |
| 2 | 4-F3CO-C6H4- | 57 | 4.3 ± 0.3 | 7.1 ± 1.1 | 105 ± 27 | 13.5 ± 0.9 |
| 3 | 3-Cl-C6H4- | 76 | 77.5 ± 1.7 | 59.8 ± 3.1 | 108 ± 28 | 22.0 ± 0.7 |
| 4 | 2-Cl-C6H4- | 80 | 20.5 ± 4.8 | 19 ± 0.9 | 185 ± 76 | 6.3 ± 1.6 |
| 5 | 4-Cl-C6H4- | 88 | 31.7 ± 0.6 | 24 ± 2.2 | 105 ± 5 | 23.8 ± 3.5 |
| 6 | 2,6-di-Cl-C6H3- | 90 | 5.3 ± 0.1 | 3.1 ± 0.9 | 171.9 ± 9.1 | 5.1 ± 4.2 |
| 7 | 3-Br-C6H4- | 85 | 16.1 ± 2.2 | 28.6 ± 5.6 | 20 ± 0.1 | 48.4 ± 2.5 |
| 8 | 2-Br-C6H4- | 83 | 31.6 ± 2.8 | 26.3 ± 5.6 | 30.7 ± 1.8 | 41.7 ± 1.8 |
| 9 | 4-Br-C6H4- | 79 | 30 ± 3.3 | 22.8 ± 1.4 | 38.3 ± 2.4 | 40.1 ± 3.5 |
| 10 | 2-F-6-Cl-C6H3- | 82 | 34.4 ± 1.8 | 8 ± 5.6 | 52.3 ± 6.7 | 21.3 ± 4.3 |
| 11 | 4-F3C-C6H4- | 74 | 11.8 ± 1.8 | 5.6 ± 1.8 | 80.5 ± 0.4 | 15.5 ± 1.5 |
| 12 | 4-Ph-C6H4- | 70 | 23.6 ± 7.0 | 26 ± 2.9 | 122.2 ± 3.1 | 11.2 ± 2.4 |
| 13 | Ph | 77 | 23.5 ± 5.9 | 18.5 ± 0.8 | 43.3 ± 4.8 | 16.7 ± 2.0 |
| 14 | 3-O2N-C6H4- | 62 | 9.1 ± 0.7 | 10.8 ± 4.7 | 19.1 ± 2.8 | 33.6 ± 3.5 |
| 15 | 2-O2N-C6H4- | 76 | 11.2 ± 2.6 | 8.3 ± 1.9 | 16.8 ± 2.4 | 25 ± 1.1 |
| 16 | 4-O2N-C6H4- | 84 | 16.8 ± 1.5 | 9.8 ± 1.0 | 24.3 ± 1.0 | 23.1 ± 1.1 |
| 17 | 4-HOOC-C6H4- | 86 | 119 ± 49 | 94.4 ± 3.1 | 332 ± 55 | 4.1 ± 1.8 |
| 18 | 4-HO-C6H4- | 69 | 30.6 ± 2.0 | 8.6 ± 0.6 | 85 ± 20 | 16.7 ± 0.9 |
| 19 | 4-iPr-C6H4- | 73 | 62.5 ± 13.6 | 34 ± 7.4 | 80.6 ± 18.8 | 2.9 ± 4.3 |
| 20 |
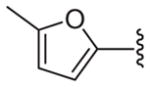
|
55 | 54.3 ± 7.3 | 51.6 ± 1.4 | 47.8 ± 3.7 | 57.8 ± 0.5 |
| 21 |
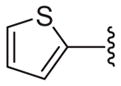
|
51 | 94 ± 5.3 | 36.9 ± 1.7 | 82.4 ± 3.4 | 11.2 ± 0.7 |
| 22 |

|
63 | 14.5 ± 0.8 | 6.0 ± 1.7 | 23.9 ± 1.2 | 28.6 ± 3.3 |
| 23 |
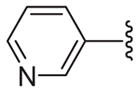
|
63 | 14.2 ± 1.0 | 3.4 ± 0.8 | 20.4 ± 5.8 | 20.8 ± 0.4 |
| α-Lapachone | N/A | N/A | 25 ± 3.8 | 16.7 ± 3.1 | 75 ± 1.4 | 7.4 ± 2.3 |
| β-Lapachone | N/A | N/A | 4.6 ± 0.5 | 6.0 ± 2.2 | 6.1 ± 0.5 | 51 ± 1.6 |
Concentration required to reduce the viability of cancer cells by 50% after 48 h of treatment with indicated compounds relative to DMSO control ± SD from two independent experiments, each performed in 8 replicates. Determined by MTT assay.
% Apoptotic Jurkat cells after 24 h of treatment with indicated compounds at the concentration of 50 μM relative to DMSO control ± SD from two independent experiments, each performed in 4 replicates. Determined by flow cytometric Annexin-V/propidium iodide assay.
