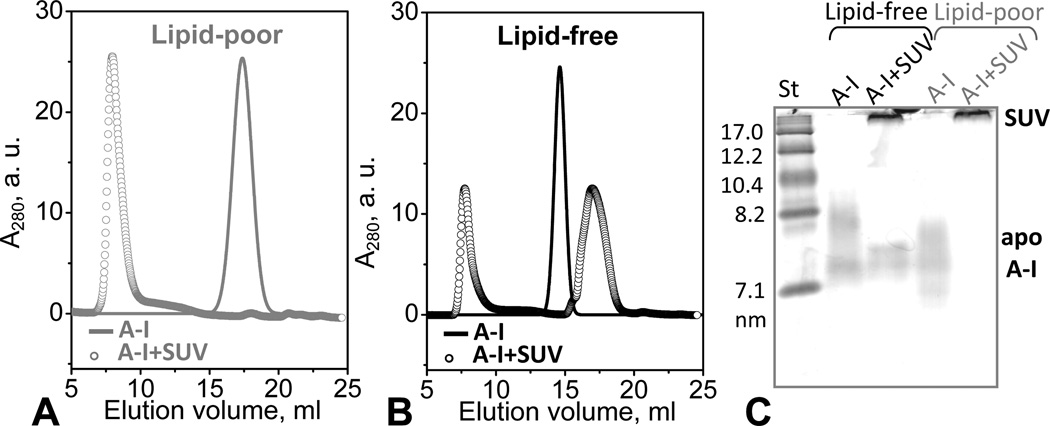
Figure 6.
Apolipoprotein adsorption to phospholipid surface. Protein-containing fractions formed upon adsorption of lipid-poor or lipid-free apoA-I to POPC SUV were detected by SEC (A, B) and NDGE (C). Protein concentration was 0.5 mg/mL in 10 mM PBS, pH 7.5; at this concentration, lipid-free apoA-I is significantly self-associated. ApoA-I:POPC molar ratio was 1:100 (open circles in A, B). Solid lines show protein in the absence of POPC SUV. (C) For NDGE (4–20 % gradient), 10 µg of protein from a 0.8 mg/mL solution were loaded per lane. Lipid-free apoA-I at this concentration is largely self-associated. Bands corresponding to apoA-I in solution or bound to SUV (d>22 nm) are indicated.