Table 1.
Preliminary Experiments and Optimization a
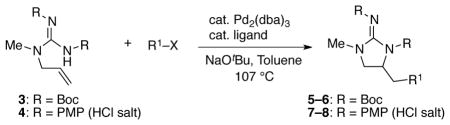
| |||||
|---|---|---|---|---|---|
| entry | substrate | R1 | ligand | product | yield (%)b |
| 1 | 3 | 2-naphthyl | Xantphos | 5 | (20–66) |
| 2 | 3 | 4-tolyl | Xantphos | 6 | (26) |
| 3 | 3 | 2-naphthyl | Nixantphos | 5 | 33 (41) |
| 4 | 3 | 4-tolyl | Nixantphos | 6 | 26 (53) |
| 5 | 3 | 2-naphthyl | Dpe-phos | – | 0 |
| 6 | 3 | 4-tolyl | Dpe-phos | – | 0 |
| 7 | 4 | 2-naphthyl | Xantphos | 7 | (74) |
| 8 | 4 | 4-tolyl | Xantphos | 8 | (65) |
| 9 | 4 | 2-naphthyl | Nixantphos | 7 | 57 (81) |
| 10 | 4 | 4-tolyl | Nixantphos | 8 | 73 (77) |
Conditions: 1.0 equiv of guanidine substrate, 1.5 equiv R–X, 2.4 equiv NaOtBu, 2 mol % Pd2(dba)3, 8 mol % ligand, toluene (0.1 M), 107 °C. Reactions of 4 were quenched with an excess of aqueous HCl (1 M) to ensure complete protonation of the guanidine.
Isolated yields. Numbers in parentheses are NMR yields based on phenanthrene as an internal standard.
