Abstract
We report on an experimental study of the role of mode of delivery and pregnancy on the architecture of vaginal elastic fibers and vaginal vault elasticity in female Sprague-Dawley rats. In primiparous rats submitted to spontaneous or Cesarean delivery and virgin rats submitted to simulated delivery, the tortuosity of elastic fibers (defined as the ratio of length to end-to-end distance) was observed to decrease when measured two days to two weeks postpartum. In addition, the measured tortuosity of elastic fibers in multiparous rats was greater than that of virgin rats. The tortuosity of elastic fibers of all rats measured at two days postpartum were found to be similar to that of multiparous rats. At two weeks postpartum the measured tortuosity of vaginal elastic fibers was indistinguishable from virgin rats, regardless of the delivery method. Borrowing from the field of polymer physics, a model is suggested that connects elastic fiber tortuosity to the resulting tension under an applied stress; fibers having high tortuosity are expected to provide less structural support than more linear, low tortuosity fibers. To probe the macroscopic effects in elasticity due to architectural changes observed in elastic fibers, we have measured the stiffness of the vaginal vault in each cohort using a pressure-infusion system. The vaginal vault stiffness of all primiparous rats measured two weeks postpartum was greater than that measured two days postpartum. In addition, the vaginal vault of virgin rats was stiffer than that of multiparous rats. These observations confirmed that vaginal vault elastic fibers undergo significant remodeling due to pregnancy and parturition, and that the complex remodeling may be a significant contributor to tissue elasticity. Remarkably, regardless of the mode of delivery or simulated tissue trauma, elastic fiber tortuosity is observed to decrease from two days to two weeks postpartum indicating the onset of repair and recovery of tissue stiffness.
Keywords: Elastic Fiber, Pelvic Organ Prolapse
1. Introduction
Elastic fibers endow tissues with the properties of resilience and recoil (Mecham, 2001). They are abundant within the endopelvic fascia (the connective tissue sheath that envelops all of the pelvic organs and maintains the vagina and uterus in their normal anatomic positions), and the fibromuscular tissue of the reproductive tract (Starcher and Percival, 1985). In most healthy adult tissues, after elastic fibers have been deposited, very little remodeling occurs (Mithieux and Weiss, 2005). Elastic fibers of the reproductive tract differ in that they undergo significant remodeling during pregnancy and post-partum involution (Woessner et al., 1963). With the recent introduction of two murine knock-out (KO) models to Lysyl Oxidase Like 1 (LOXL-1) and Fibulin 5 (FBLN 5) that develop pelvic organ prolapse (POP) due to aberrant elastic fiber homeostasis an important physiologic link between elastic fibers and POP has been observed (Liu et al., 2006). In light of these findings, and the long held belief that pregnancy and the process of labor and delivery are associated with the development of POP (Ricci, 1950; Emge and Durfee, 1966), investigators have recently focused on understanding how pregnancy and parturition influence elastic fiber remodeling.
Drewes et al. studied the regulation of elastic fiber synthesis and assembly in the vaginal wall of mice during pregnancy and after vaginal delivery. They showed that in the immediate postpartum a burst in the protein expression of FBLN 5 and tropoelastin occurs (Drewes et al., 2007). It has also been shown that a marked increase in matrix metalloproteinase (MMP)-2 and -9 activity occurs in the vaginal wall tissue of wild type mice after vaginal delivery during the immediate postpartum (Rahn et al., 2008; Wieslander et al., 2008). These findings have led to the notion that the maintenance of pelvic organ support may hinge on a delicate balance of elastic fiber remodeling, which involves elastic fiber synthesis and degradation, and that an imbalance in this process could lead to the development of POP. To test this hypothesis, Drewes et al. injected elastase into the posterior vaginal wall of early postpartum wild type mice, resulting in the development of posterior wall prolapse in the elastase treated animals but not in the saline or inactivated elastase treated animals. In addition, elastic fibers in the elastase treated animals were shortened and frayed compared to controls (Drewes et al., 2007). Rahn et al. tested this hypothesis in an alternate approach by exposing nulliparous FBLN 5 KO, FBLN heterozygotes and wild type mice to vaginal balloon distention at six weeks of age (prior to the spontaneous onset of POP in FBLN 5 KO mice) and found that only the FBLN 5 KO mice developed POP. They also noted that vaginal distention resulted in elastic fibers appearing fragmented and disrupted(Rahn et al., 2008). Furthermore, Budatha et al., using a knock-in mouse with a mutant arginine/glycine/aspartic acid motif (RGD-a sequence known to mediate binding to cell surface integrin receptors) and a double KO FBLN 5 MMP-9 mouse showed that the development of POP was correlated to the presence of aberrant elastic fibers and the up-regulation of MMP-9 (Budatha et al., 2011). The work of these authors has affirmed the link between aberrant elastic fiber remodeling, vaginal delivery and POP in an animal model. However, little is known about the effect of cesarean delivery on elastic fiber remodeling in reproductive tract tissues.
Cesarean delivery accounts for nearly one third of all U.S. deliveries (Martin et al., 2012), and has been shown to be protective from the onset of pelvic floor disorders. For example, a population based study by Quiroz et al. involving 290 females demonstrated Cesarean births in women over the age of 40 were not associated with prolapse while a single vaginal birth significantly increased the odds of prolapse (defined as descent to or beyond the hymen)(Quiroz et al., 2010). Similarly, Leijonhufvud et. al. have shown that vaginal childbirth was associated with a significant increase of stress urinary incontinence and pelvic organ prolapse surgery later in life compared with only having cesarean delivery (Leijonhufvud et al., 2011).
Here, we focus on a study of the effect of mode of delivery on elastic fiber remodeling in the vaginal wall of Sprague-Dawley rats, and changes in vaginal tissue elasticity. Specifically, we have investigated the morphology of elastic fibers in the vaginal tissues of rats submitted to different modes of delivery. Using image analysis software developed in MATLAB we have directly measured the length and tortuosity of elastic fibers (defined as the ratio of length to end-to-end distance); fibers with high tortuosity are expected to provide less support to tissue. To assess tissue function we custom designed an ex-vivo pressure-infusion system that allows for measuring the stiffness of the vaginal vault. In light of the data discussed above, we have hypothesized that tissue exposed to birth trauma (i.e., simulated vaginal delivery and spontaneous vaginal delivery) will undergo poor remodeling, exhibiting highly tortuous elastic fibers and reduced stiffness compared to virgin rats. We believe that data derived from this study will aid in the general understanding of how elastic fiber remodeling is affected by pregnancy and parturition and will provide additional insight into how these events may contribute to the development of POP.
2. Materials and Methods
2.1. Preparation of tissues
All study protocols were approved by the Animal Institute Committee of the Albert Einstein College of Medicine. The study utilized 36 adult female Sprague-Dawley rats separated into the following cohorts: virgin rats submitted to simulated vaginal delivery by vaginal balloon dilation, and primiparous rats allowed to undergo spontaneous vaginal delivery or Cesarean delivery. In the simulated delivery group, a 10 French Foley catheter was inserted into the vagina under anesthesia and inflated with 2.5 cc of water. In this procedure, the catheter was hung off the laboratory bench for 180 minutes and then removed while still inflated. Pregnant rats were allowed to undergo spontaneous vaginal delivery, with pups removed from the cage upon completion of delivery, while others were submitted to Cesarean delivery on gestation day 21 as follows: under anesthesia, a small vertical incision was made in the lower ventral abdominal wall allowing for delivery of the gravid uterine horns. Through longitudinal incisions in the uterine myometria, rat pups and their placentas were removed, the horns returned to the abdominal cavity and the ventral incision closed with permanent suture in two layers (fascia/muscle and skin). Animals were sacrificed in a carbon dioxide chamber two days and two weeks after their respective intervention, followed by surgical resection of the entire genital tract. Other rat groups analyzed were virgin rats between 12 and 15 weeks of age and multiparous rats between 9 and 12 months of age with parity ranging from 3 to 7 deliveries.
2.2. Microscopy
Animal sacrifices were carried out in a sealed chamber according to protocol by carbon dioxide inhalation. For each animal, the reproductive tract was excised en bloc and placed in formalin for 24 hours then transferred to 70% ethanol and stored at −22°C until sectioned. Careful attention was paid to the orientation (distal, proximal, left, and right with respect to the vaginal vault) of the sample throughout the sectioning process. Segments of the upper vagina (vaginal fornices) were excised and suspended in a 30% sucrose solution (m/v) in phosphate buffered saline at 0°C overnight before sectioning. All tissue samples were sectioned at a thickness of 16 μm using a Leica CM1850 cryostat. The samples of upper vaginal tissue were then mounted on the cryostat chuck using Instrumedics Cryo-Gel (CAT 475237). The orientation of the sample was also taken into account while sectioning and plating the samples. Samples were stained using accustain (Sigma-Aldrich HT25A-1KT) using the following procedure. The slides were first placed in the working elastin stain solution for approximately 11 minutes which is comprised of 20 ml of hematoxylin solution, 3 ml of ferric chloride solution, 8 ml of Weigert's iodine solution, and 5 ml of deionized water. Following this step, the sections were rinsed in deionized water and placed in the working ferric chloride solution, which includes 3 ml of ferric chloride and 37 ml of deionized water, for 2 minutes. Next, the sections were rinsed with deionized water, followed by a rinse in 95% ethanol to remove any remaining iodine, and then rinsed again in deionized water. Subsequently, the samples are submerged in Van Giesen solution for 90 seconds, rinsed with 95% ethanol, and finally dehydrated with xylene until the cover slips are mounted onto the slides using Eukitt quick-hardening mounting medium. All slides were left to dry overnight before imaging. The samples were placed under a monocular microscope with a built in digital camera. Images of the distal, proximal, right, and left regions were taken for each of the outer, middle, and inner thirds at 40x for every sample. The images were then analyzed using software we have developed in our laboratory (available upon request) using MATLAB, allowing for the measurement of the length and tortuosity of individual fibers. We tested our software on a number of simple geometries (half circle, L-shape, straight line, and V-shapes) that were sketched by hand and confirmed the measured tortuosity determined with the software agreed with calculated tortuosity. The number of fibers used for the measurement of the fiber tortuosity and length are shown in parenthesis in Tables 1 and 2. Five individuals who were blinded from the expected results performed the measurements of the fiber tortuosity and length. Two rats in each group were used, and the experimentally measured tortuosity and length of elastic fibers was determined from the weighted average of the measurements on two rats.
Table 1.
Mean tortuosity of elastic fibers observed in the vaginal wall of Sprague-Dawley rats having undergone various modes of delivery, as described in the text, for two distinct sets of rats. The numbers shown in parenthesis represent the total number of fibers over which the measurements were taken. The results of a t-test are shown in the forth column indicating the probability that the measurements are statistically different, comparing two days to two weeks postpartum rats (simulated, spontaneous and Cesarean cohorts) and between the multiparous and virgin rats.
| Mean Elastic Fiber Tortuosity | 2 Days | 2 Weeks | t-test [%] |
|---|---|---|---|
|
| |||
| Simulated | 1.2314 ± 0.0031 (2978) | 1.1218 ± 0.0021 (1754) | 99.9 |
|
| |||
| Spontaneous | 1.2974 ± 0.0041 (1762) | 1.1733 ± 0.0033 (1616) | 99.9 |
|
| |||
| Cesarean | 1.2321 ± 0.0038 (1853) | 1.1372 ± 0.0038 (1600) | 99.8 |
|
| |||
| Multiparous | 1.3598 ± 0.0094 (2635) | 99.8 | |
| Virgin | 1.1912 ± 0.0064 (2340) | ||
Table 2.
Mean length of elastic fibers observed in the vaginal wall of Sprague-Dawley rats having undergone various modes of delivery, as described in the text, for two distinct sets of rats. As in Table 1, the numbers in parenthesis represent the total number of fibers over which the measurements were taken. The results of a t-test are shown in the forth column, indicating the probability that the measurements are statistically different comparing two days to two weeks postpartum rats (simulated, spontaneous and Cesarean cohorts) and between the multiparous and virgin rats.
| Mean Fiber Length | 2 Days [μm] | 2 Weeks [μm] | t-test [%] |
|---|---|---|---|
|
| |||
| Simulated | 45.07 ± 5.33 (2978) | 75.38 ± 6.59 (1754) | 98.1 |
|
| |||
| Spontaneous | 56.67 ± 3.82 (1762) | 58.34 ± 4.98 (1616) | 28.5 |
|
| |||
| Cesarean | 56.77 ± 5.76 (1853) | 59.95 ± 5.46 (1600) | 49.4 |
|
| |||
| Mulitparous | 54.64 ± 5.89 (2635) | 72.5 | |
| Virgin | 48.72 ± 5.31 (2340) | ||
2.3. Pressure-Infusion System
For measuring the volumetric change with pressure, a separate set of rats were used (different than those used for the microscopy work). Two rats in each group were used, except in the case of the spontaneous delivery cohorts where 4 rats were used, and the experimentally measured value of h (or κ) was determined from the average of the measurements on two rats. The genital tract of each rat was harvested en bloc immediately following sacrifice, and placed in Krebs-Henseleit buffer (124 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 0.6 mM KH2PO4, 10 mM C6H12O6 and 25 mM NaHCO3). The endocervix was ligated with a nylon suture to prevent transmission of fluid to the uterine horns. The vaginal introitus was secured with a nylon ligature to the infusion tubing and then placed within the dual chamber well. The inner chamber of the well was filled with Krebs-Henseleit buffer, and the outer chamber was filled with circulating water at 37° C. A schematic of the pressure-infusion system is shown in Figure 1. The infusion pump, attached to one arm of the T shaped tubing was set at a rate of 0.05 ml/min and instilled room temperature Krebs-Henseleit buffer. A pressure transducer attached to the other arm of the T shaped tubing, was used for measuring the change in pressure using a PowerLab 4/30 data acquisition system (ADInstruments, Colorado Springs, CO). All pressure-infusion measurements were performed immediately after harvesting the genital tract.
Figure 1.
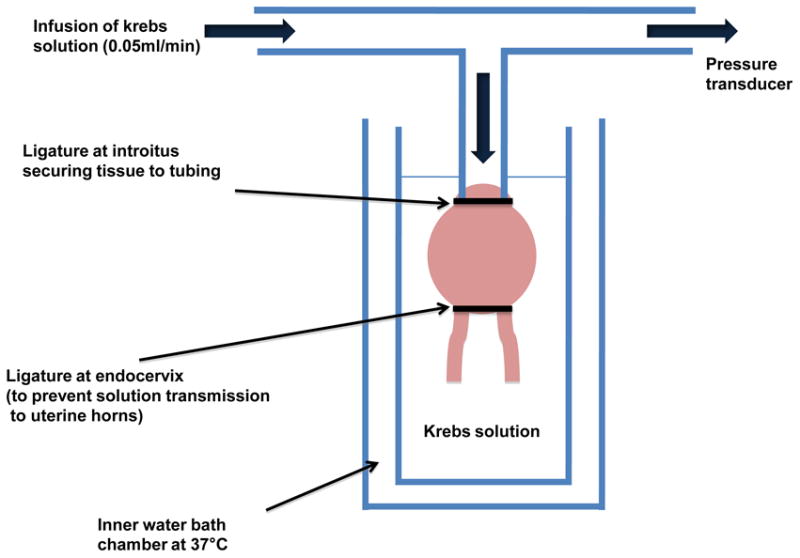
Schematic of the pressure-infusion system used in this study. In the experiments, the endocervix was ligated with a nylon suture to prevent transmission of fluid to the uterine horns, while the vaginal introitus was secured with a nylon ligature to the infusion tubing to create a hermetic seal. The inner chamber of the bath was filled with Krebs-Henseleit buffer and the outer chamber was filled with circulating water at 37°C. A stepper motor was used to fill the vaginal vault at a rate of 0.05ml/min, and the resulting change in the vaginal vault pressure was measured using a transducer.
2.4. Statistical Methods
Measurements of the elastic fiber tortuosity and length from each cohort were averaged and a standard error of the mean (SEM) was determined. For the pressure-infusion studies measured values of κ and h were determined by five repeated measures in each cohort and an SEM was calculated. A T-test was implemented under the assumption that our data followed a normal distribution, and using the observed standard deviations in each cohort. Probabilities of the null hypothesis were determined indicating the level of significance of our data.
3. Results and Discussion
Representative images of the stained upper vaginal sections from our study are shown at 40x magnification in Figures 2 through 5. In the figures elastic fibers are stained black, collagen is stained red, and smooth muscle is stained yellow. Figure 2 shows an example stained section of the upper vaginal wall of a Sprague-Dawley rat having undergone simulated delivery two days (Figure 2 left) and two weeks (Figure 2 right) postpartum. Similarly, Figure 3 shows example stained sections from rats that had undergone spontaneous delivery and Figure 4 sections from rats that underwent Cesarean delivery. Figure 5 highlights example stained sections of a multiparous Sprague-Dawley rat (Figure 5 left) and that of a virgin rat (Figure 5 right). A careful examination of the images we accumulated (in total approximately 75 images were accumulated for each cohort) indicated that elastic fibers in the simulated, spontaneous, and Cesarean delivery rats appeared more linear when comparing sections from two days to two weeks postpartum. The elastic fibers of the virgin rat also appear more linear than those of the multiparous rats in addition to the fibers observed in the simulated, spontaneous, and Cesarean delivery rats two days postpartum. While the representative images shown may indicate some difference in collagen, smooth muscle and elastic fiber content, our overall histological work did not reveal any significant variation across the different groups or within a group.
Figure 2.
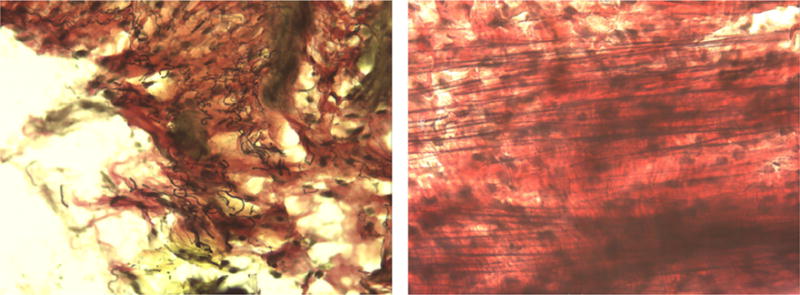
Representative histological section (at 40 ×) from the upper vaginal wall (left) two days and (right) two weeks postpartum of simulated vaginal delivery Sprague-Dawley rats. Elastic fibers are stained black, collagen is stained red, and smooth muscle is stained yellow.
Figure 5.
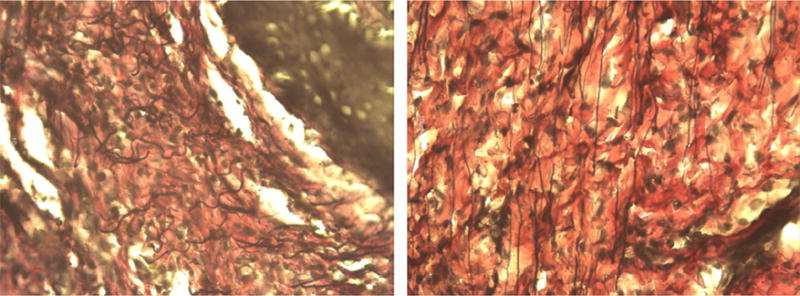
Representative histological section (at 40X) from the upper vaginal wall of a multiparous (left) and virgin (right) Sprague-Dawley rat. Elastic fibers are stained black, collagen is stained red, and smooth muscle is stained yellow.
Figure 3.
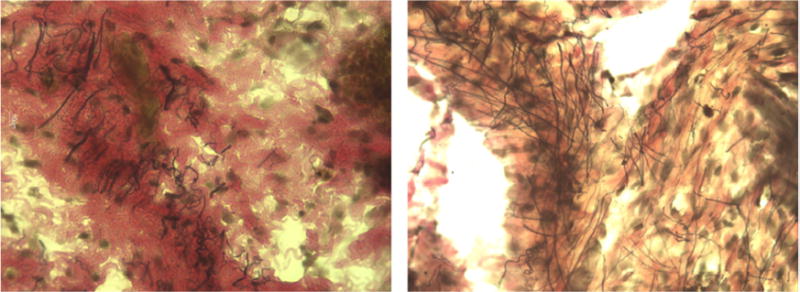
Representative histological section (at 40X) from the upper vaginal wall (left) two days and (right) two weeks postpartum of spontaneous vaginal delivery Sprague-Dawley rats. Elastic fibers are stained black, collagen is stained red, and smooth muscle is stained yellow.
Figure 4.
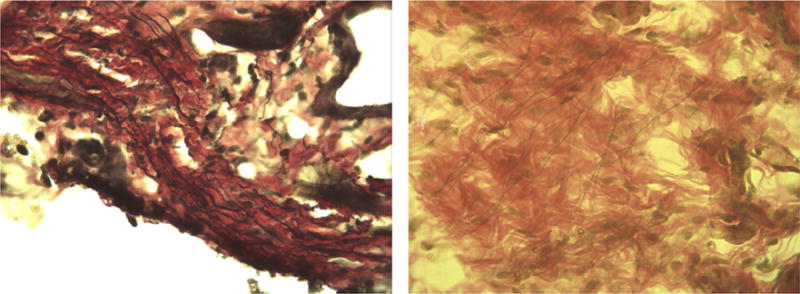
Representative histological section (at 40X) from the upper vaginal wall, (left) two days and (right) two weeks postpartum of Cesarean delivery Sprague-Dawley rats. Elastic fibers are stained black, collagen is stained red, and smooth muscle is stained yellow.
The tortuosity of a fiber, which we denote τ hereafter, is defined as the integrated path length divided by the end to end distance (Boas, 2006). For a two dimensional image, the tortuosity is given by the relationship
| (1) |
In the above expression, x and y denoting pixel coordinates and Δx and Δy the end to end distances of a given fiber in the image. Using the above definition we have measured the vaginal elastic fiber tortuosity of the reproductive tract of all rats in this study. Table 1 summarizes the measurements of the average fiber tortuosity collected from Sprague-Dawley rats two days and two weeks postpartum following various modes of delivery. The table details the number of fibers (shown in parenthesis in each row) over which the statistics were accumulated. The numbers reported were determined by performing an average over fibers from the inner, middle, and outer third of the upper vaginal wall. We found no significant variation across each layer, or distal, proximal to the vagina; this measurement was quite challenging given the small size of the tissues studied and thickness of each section. The error bar shown in Tables 1 and 2 represents the standard error in the weighted mean. The data shown in Table 1 indicates a decrease in tortuosity from two days to two weeks for Cesarean, simulated, and spontaneous modes of birth. Virgin rats were observed to have the smallest tortuosity, while elastic fibers from multiparous rats had the highest tortuosity. Table 2 lists measurements of the length of the fibers measured two days and two weeks postpartum following the various modes of delivery. No statistically significant changes were observed when comparing the average fiber length from two days to two weeks postpartum within a group, except for the case of simulated delivery. This last finding may suggest that elastic fiber remodeling after direct trauma differs in some way from that following pregnancy.
Borrowing from the field of polymer physics, the tortuosity of the elastic fiber should correlate with the tension it can provide under stress and consequently alter the elasticity of the tissue. In fact studies of radiation exposure to the skin have revealed a qualitative increase in elastic fiber tortuosity, which was shown to be correlated to a decrease in elastic properties of the skin and the onset of actinic elastosis (Imayama et al., 1994; Kobayashi et al., 2005; Imayama et al., 1994; Tsukahara et al., 1999). A flexible polymer chain may be modeled as a superposition of adjoined monomers, which are capable of reorienting in any given direction allowing for a linear to tortuous chain. The result is that the polymer tension (f) is proportional to its end to end length < r >
| (2) |
In the above expression, N is the number of monomers, T is the temperature, and a the individual monomer length (De Gennes, 1979). Thus linear fibers (τ = 1 and < r > is a maximum) would be expected to provide more support to the tissue compared with highly tortuous (τ > 1) fibers. In other words, highly tortuous fibers would require a reduction in tortuousity (i.e. straightening out) before providing the same tension under a given strain.
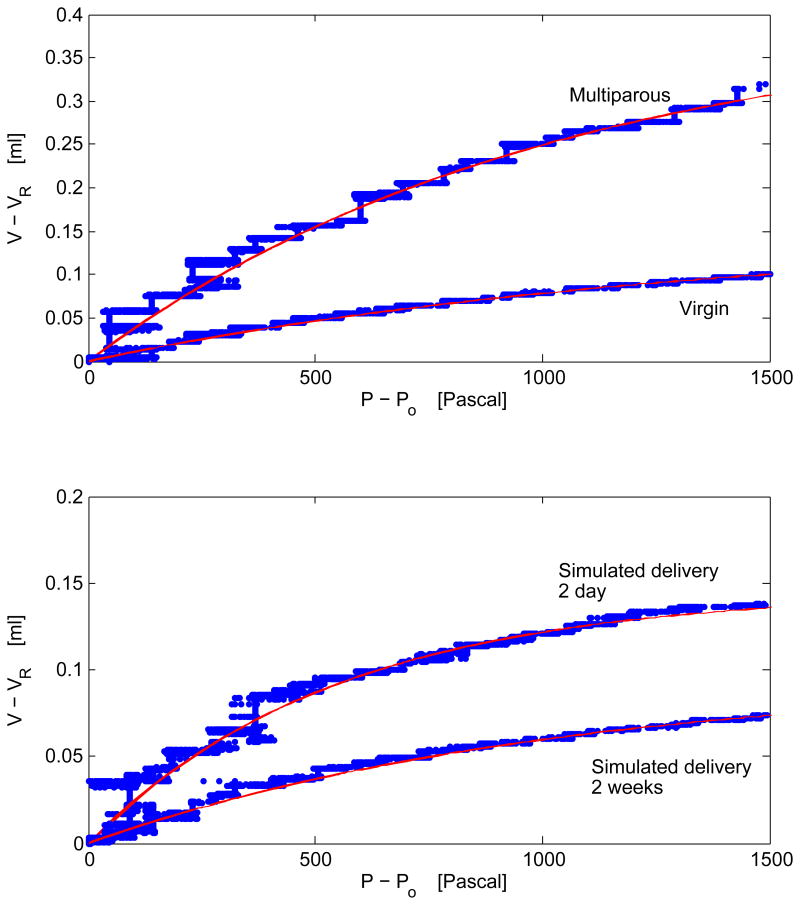
Using a pressure infusion system, we measured the change in the vaginal vault volume for each of the rats studied under an applied pressure as detailed in Experimental Procedure. In all cases studied, we experimentally observed that the change in volume with pressure followed an exponential dependence. Example pressure-volume curves are shown in Figure 6 for multiparous and virgin and two day and two week simulated delivery rats. The observed trend is reminiscent of measurements of the change in volume with pressure observed in the lung (Salazar and Knowles, 1964; Greaves et al., 1980; Schroter, 1980; Hu et al., 2010). Below we apply a similar mathematical treatment to quantify the stiffness of the vaginal vaults in our study, and for completeness, we review the salient features of the model introduced by Salazar and Knowles (Salazar and Knowles, 1964).
Figure 6.
Representative pressure-volume curves of the vaginal vaults of (top) multiparous and virgin and (bottom) simulated two day and two week postpartum Sprague-Dawley rats. As discussed in the text, the change in volume with pressure is observed to follow an exponential. In all cases studied, the change in volume with pressure was fit to a mathematical model introduced in pressure-volume studies of the lung (Salazar and Knowles, 1964), allowing for a determining the stiffness of the vaginal vault; resulting parameters from the fit are documented in Table 3. The red lines shown in each case represent a 22 fit of equation 4 to the experimental data. The slope of each curve is quantified by the parameter h (or κ). One should note that the maximal volume (Vo), the asymptotic value achieved in the pressure-volume curves shown, varies across rats with multiparous rats exhibiting the largest value.
Physically one would expect that the change in volume with pressure, dV/dP, should decrease with increasing pressure and approach zero as the maximum vault volume, V0, is achieved. This may be mathematically formulated by relationship
| (3) |
where κ is a constant of proportionality having units of inverse Pascal and is discussed below. Taking VR as the initial volume (the resting vaginal vault volume at atmospheric pressure P0) the above expression may be integrated. The resulting expression is
| (4) |
Following the notation introduced by Salazar and Knowles (Salazar and Knowles, 1964) the requisite pressure to achieve one-half of the maximal volume is given by the parameter
| (5) |
Had the experimental data shown in figure 6 indicated a linear change in volume with pressure, one would have been able to simply extract the bulk modulus of the tissue β = dV/dP, a constant familiar from material science. However, the change in volume with pressure, shown in Figure 6, is highly nonlinear. Although the constant h has units of Pascal it is not the same as the bulk modulus, but is related to the stiffness or pliability of the tissue; an increase (decrease) in the constant h dictates an increase (decrease) in the requisite pressure to achieve one-half of the maximum volume(Salazar and Knowles, 1964). One point worth mentioning in our modeling of the experimental data is that the measurements are insensitive to any fast dynamical changes to the tissue as the sampling time on our digitizer that read the changes in pressure was set to 0.01 seconds. Nevertheless, the data in all cases given this sampling time appeared to be well described by an exponential given in equation 4.
Table 3 highlights the measured values of κ and h determined for each cohort studied. As discussed in Section 2, the data shown were accumulated on two separate rats (four rats in the vaginal delivery cohort) and the vaginal vault in each case was measured five times. Referring to Table 3, the experimental data indicate that h (or equivalently κ) increases when measured two days to two weeks postpartum. The numerical value of h for the two weeks postpartum spontaneous, Cesarean, and simulated delivery rats all appear similar, and more notably, the change observed from two days to two weeks postpartum appears to be similar across all groups within our experimental uncertainty. In addition, the measured value of h for the virgin rats appears to be larger than that of the multiparous rats.
Table 3.
Experimentally determined values of κ and h of the vaginal vaults of Sprague-Dawley rats. In each group two rats were used, except in the spontaneous delivery groups where 4 rats were used for both the two day and two week postpartum measurements. The parameters were determined by fitting equation 4 to the measured change in volume with applied pressure for each cohort. As discussed in the text, h is a measure stiffness of the tissue and represents the pressure required to reach one-half of the maximum volume (Salazar and Knowles, 1964). The values shown were obtained from measurements on two rats within each group. The results of a t-test are shown, which quantify the probability that the observations are statistically different comparing two days to two weeks post-partum rats (simulated, spontaneous and Cesarean cohorts) and between the multiparous and virgin rats.
|
|
|
t-test [%] | |||
|---|---|---|---|---|---|
| Simulated 2 Days | 5.06 ± 1.40 | 3.51 ± 0.97 | 89.17 | ||
| Simulated 2 Weeks | 8.51 ± 3.21 | 5.90 ± 2.23 | |||
| Spontaneous 2 Days | 4.62 ± 0.74 | 3.21 ± 0.51 | 98.89 | ||
| Spontaneous 2 Weeks | 9.67 ± 0.16 | 6.70 ± 0.11 | |||
| Cesarean 2 Days | 5.32 ± 1.17 | 3.69 ± 0.81 | 89.37 | ||
| Cesarean 2 Weeks | 8.50 ± 2.97 | 5.89 ± 2.06 | |||
| MP | 5.41 ± 2.25 | 3.75 ± 1.56 | 88.49 | ||
| Virgin | 8.36 ± 2.14 | 5.79 ± 1.48 |
Previous studies of the biomechanical adaptions of the rat vagina and supportive tissue due to pregnancy appear to be in good agreement with the findings reported in this work. Specifically, a recent study that focused on the biomechanical properties of the vagina and supportive tissues under loading conditions that simulate downward distension reported that the linear stiffness appeared to reduce at stages of mid- and late-pregnancy and immediately after vaginal or abdominal delivery in comparison to virgin rats. However, 4 weeks following delivery, a recovery of the tissue linear stiffness was documented for both vaginal and abdominal deliveries, which approached the measured values for virgin rats (Lowder et al., 2007). A similar finding was reported in a more recent work by the same group wherein the passive and active mechanics of the rat vagina was studied. The tangent modulus of 4-week postpartum rats was similar to that of virgin rats, while late- and mid-pregnancy rats as well as 2 hour-postpartum rats showed statistically lower tangent moduli(Feola et al., 2011). These findings emphasize the notion that the remodeling of the extracellular matrix is important for reducing the risk of maternal injury during delivery and that during the late postpar-tum period a recovery of the tissue occurs allowing for the recovery of tissue stiffness. Our findings, while using a different experimental protocol, showed analogous results and also showed that tissue stiffness in cohorts that underwent simulated delivery recovered after 2 weeks postpartum; this finding may suggest that pregnancy itself is not a requirement to stimulate elastic fiber repair.
A number of studies on both rat and human prolapsed tissues have shown that the architecture of elastic fibers may directly impact the macroscopic properties of tissue. For example, LOXL1 deficient mice show a substantial accumulation of elastin monomers and the absence of cross-linked products; the tissues in this study were documented to tear at the slightest variation of any force(Liu et al., 2006). A study of 59 women showed an absence of or fragmented elastic fibers in biopsies from women with POP(Goepel, 2008). A recent study of uterosacral ligament biopsies from women with and without POP demonstrated a reduction in elastin content in women with POP and that the mRNA levels of LOXL1 was significantly reduced in women with POP(Klutke et al., 2008). In a study of fibulin-3 and-5 deficient mice that developed POP, elastic fibers were found to be grossly fragmented, tortuous and tangled in the adventitial layer of the vaginal wall(Rahn et al., 2009). In studies of female stress urinary incontinence, irregular fragmented distributions of elastin were documented in women with hypotonic urethra, emphasizing the important scaffolding role of the elastic fibers in reproductive tissues. (Goepel and Thomssen, 2006).
A key finding of our study is that mode of delivery in primiparous rats does not appear to have a differential effect on the macroscopic architecture of elastic fibers, or on their functionality whether assessed early or late postpar-tum. It may be that mode of delivery is not the central driving force behind connective tissue aberrations that result in POP or other forms of pelvic floor dysfunction. The known changes in connective tissues due to pregnancy, regardless of mode, may be a key factor that influences elastic fiber remodeling. However, one must also consider a shortcoming of this study: while the Sprague-Dawley rats used in this study are ideal experimental animals given their low cost to purchase and house, ease of handling and short gestation period, they do not develop pelvic organ prolapse. In light of this fact, we have implemented a custom made pressure infusion system to calculate a measure of tissue stiffness following different modes of birth as well as direct trauma to the tissue. One might argue that the measurement of tissue stiffness may be considered a proxy for POP as it provides an assessment of the tissue behavior relative to the organization of its elastic fiber content. In fact, it has been shown that the biomechanical properties of prolapsed vaginal wall tissue are similar to those observed during pregnancy(Rahn et al., 2008), a finding our data supports. Nevertheless, to better determine the exact contribution of elastic fibers to tissue stiffness, one might consider analyzing the structural characteristics of the other major components of the extracellular matrix (i.e., collagen and/or smooth muscle). Additionally, to gain greater insight into elastic fiber functionality, further studies using electron microscopy and nuclear magnetic resonance spectroscopy methodology may probe the microstructure of the elastic fiber including its elastin core and microfibrillar scaffolding following different modes of birth.
Although mode of delivery did not result in measurable differences in elastic fiber structure or function, we have documented a significant difference between both virgin rats and two week postpartum rats compared with multiparous rats. The two factors most likely contributing to these findings are differences in age and parity; the multiparous rats used in this study were on average between nine and twelve months of age and delivered three to seven litters, while the virgin rats and primiparous rats were between twelve and fifteen weeks of age, and at most delivered one litter. When considering that the two day postpartum rats had similar elastic fiber structure and function as the older, multiparous rats, one might speculate that advanced age, increasing parity, or both may lead to compromised elastic fiber remodeling. These factors were considered in a rat study by Rundgren who found that multiparty exerted a greater influence on the biomechanical properties of vaginal tissue than advanced age (Rundgren, 1974).
4. Conclusions
We have measured the tortuosity of elastic fibers found in the fibromuscu-lar connective tissue of the vaginal wall in cohorts of virgin and multiparous Sprague-Dawley rats as well as rats submitted to simulated, spontaneous, or Cesarean delivery. The tortuosity of elastic fibers was observed to decrease when measured from two days to two weeks postpartum in each intervention group studied, while multiparous rats were observed to have the highest tortuosity. Within our experimental uncertainty, we found that the elastic fiber tortuosity of each of our delivery cohorts at two weeks postpartum was similar to that of virgin rats. The histological methodology implemented in this study focused solely on the morphology of elastic fibers. A more detailed microscopic study of collagen and elastin structure is underway in our laboratory. Using a pressure infusion system, we measured the change in volume with pressure of the vaginal vault and correlated the observed architectural changes of the elastic fiber to tissue elasticity. For each group studied, we observed an exponential change in the pressure-volume curve. A mathematical model was implemented, similar to that used in pressure-volume studies of the lung, allowing for the determination of the requisite pressure to achieve one-half of the maximum volume, h, a physical parameter that is related to the stiffness of the tissue. The rat vaginal vault from the delivery cohorts all indicated an increase in tissue stiffness two weeks postpartum and the change across each of the groups appears to be similar within our experimental uncertainty. More notably for each of the two week primiparous groups, we found that tissue stiffness was similar to that of virgin rats and the numerical value of h of two day simulated, spontaneous, and Cesarean rats was indistinguishable from that of multiparous rats.
Highlights.
The tortuosity of elastic fibers of vaginal tissues in female Spraque-Dawley rats has been directly measured.
In primiparous Cesarean, simulated and spontaneous delivery rats we observe a decrease in elastic fiber tortuosity when determined two days to two weeks postpartum.
The tortuosity of multiparous rats was greater than that of virgin rats and all two week primiparous rats.
The stiffness of the vaginal vault was measured and observed to correlate with differences in elastic fiber tortuosity within and across each cohort of rats studied.
Acknowledgments
G.S. Boutis acknowledges support from the National Institute of General Medical Sciences of the National Institutes of Health under grant number SC1GM086268 and an Enhanced PSC-CUNY Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boas M. Mathematical Methods in the Physical Sciences. Vol. 2. Wiley; 2006. [Google Scholar]
- Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. The Journal of Clinical Investigation. 2011;121:2048. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennes P. Scaling Concepts in Polymer Physics. Cornell University Press; 1979. [Google Scholar]
- Drewes P, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis S, Keller P, Word R. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. The American Journal of Pathology. 2007;170:578. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feola A, Moalli P, Alperin M, Duerr R, Gandley RE, Abramowitch S. Impact of pregnancy and vaginal delivery on the passive and active mechanics of the rat vagina. Annals of Biomedical Engineering. 2011;39:549–558. doi: 10.1007/s10439-010-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepel C. Differential elastin and tenascin immunolabeling in the uterosacral ligaments in postmenopausal women with and without pelvic organ prolapse. Acta Histochemica. 2008;110:204–209. doi: 10.1016/j.acthis.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Goepel C, Thomssen C. Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochemica. 2006;108:441–445. doi: 10.1016/j.acthis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Greaves I, Colebatch H, et al. Elastic behavior and structure of normal and emphysematous lungs post mortem. The American Review of Respiratory Disease. 1980;121:127. doi: 10.1164/arrd.1980.121.1.127. [DOI] [PubMed] [Google Scholar]
- Hu Q, Shifren A, Sens C, Choi J, Szabo Z, Starcher BC, Knutsen RH, Shipley JM, Davis EC, Mecham RP, et al. Mechanisms of emphysema in autosomal dominant cutis laxa. Matrix Biology. 2010;29:621–628. doi: 10.1016/j.matbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama S, Nakamura K, Takeuchi M, Hori Y, Takema Y, Sakaino Y, Imokawa G. Ultraviolet-b irradiation deforms the configuration of elastic fibers during the induction of actinic elastosis in rats. Journal of Dermatological Science. 1994;7:32–38. doi: 10.1016/0923-1811(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Klutke J, Ji Q, Campeau J, Starcher B, Carlos Felix J, Stanczyk FZ, Klutke C. Decreased endopelvic fascia elastin content in uterine prolapse. Acta Obstetricia et Gynecologica Scandinavica. 2008;87:111–115. doi: 10.1080/00016340701819247. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kobayashi K, Hosumi N, Tanino R. Ultrasonic tissue characterization of photodamaged skin by scanning acoustic microscopy. Tokai Journal of Experimental and Clinical Medicine. 2005;30:217–225. [PubMed] [Google Scholar]
- Leijonhufvud Å, Lundholm C, Cnattingius S, Granath F, Andolf E, Altman D. Risks of stress urinary incontinence and pelvic organ prolapse surgery in relation to mode of childbirth. American Journal of Obstetrics and Gynecology. 2011;204:70–e1. doi: 10.1016/j.ajog.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. The American Journal of Pathology. 2006;168:519–528. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstetrics & Gynecology. 2007;109:136–143. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Mathews T. Births: Final data for 2009. National Vital Statistics Reports 61. 2012 [PubMed] [Google Scholar]
- Mecham R. Overview of extracellular matrix. Current Protocols in Cell Biology. 2001:10–1. doi: 10.1002/0471143030.cb1001s00. [DOI] [PubMed] [Google Scholar]
- Mithieux SM, Weiss AS. Elastin. Advances in Protein Chemistry. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- Quiroz LH, Muñoz A, Shippey SH, Gutman RE, Handa VL. Vaginal parity and pelvic organ prolapse. The Journal of Reproductive Medicine. 2010;55:93. [PMC free article] [PubMed] [Google Scholar]
- Rahn D, Ruff M, Brown S, Tibbals H, Word R. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2008;198:590–e1. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn DD, Acevedo JF, Roshanravan S, Keller PW, Davis EC, Marmorstein LY, Word R. Failure of pelvic organ support in mice deficient in fibulin-3. The American Journal of Pathology. 2009;174:206–215. doi: 10.2353/ajpath.2009.080212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundgren A. Physical properties of connective tissue as influenced by single and repeated pregnancies in the rat. Acta Physiologica Scandinavica Supplementum. 1974;417:1. [PubMed] [Google Scholar]
- Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. Journal of Applied Physiology. 1964;19:97–104. doi: 10.1152/jappl.1964.19.1.97. [DOI] [PubMed] [Google Scholar]
- Schroter R. Quantitative comparisons of mammalian lung pressure volume curves. Respiration Physiology. 1980;42:101–107. doi: 10.1016/0034-5687(80)90107-3. [DOI] [PubMed] [Google Scholar]
- Starcher B, Percival S. Elastin turnover in the rat uterus. Connective Tissue Research. 1985;13:207–215. doi: 10.3109/03008208509152400. [DOI] [PubMed] [Google Scholar]
- Tsukahara K, Takema Y, Fujimura T, Moriwaki S, Kitahara T, Imayama S, Imokawa G, et al. All-trans retinoic acid promotes the repair of tortuosity of elastic fibres in rat skin. British Journal of Dermatology. 1999;140:1048–1053. doi: 10.1046/j.1365-2133.1999.02902.x. [DOI] [PubMed] [Google Scholar]
- Wieslander C, Marinis S, Drewes P, Keller P, Acevedo J, Word R. Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biology of Reproduction. 2008;78:521–528. doi: 10.1095/biolreprod.107.063024. [DOI] [PubMed] [Google Scholar]
- Woessner J, et al. Formation and breakdown of collagen and elastin in the human uterus during pregnancy and post-partum involution. Biochemical Journal. 1963;89:75. doi: 10.1042/bj0890075. [DOI] [PMC free article] [PubMed] [Google Scholar]