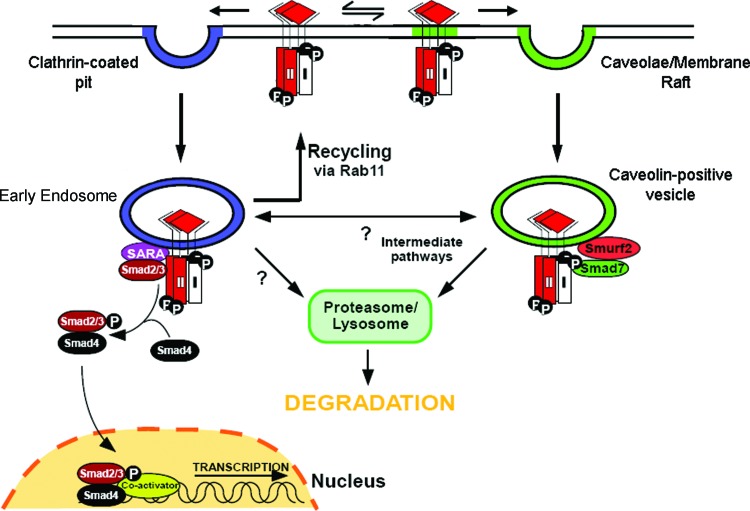
Figure 4.
Regulation of TGF-β signaling by clathrin-dependent and -independent endocytosis. TGF-β receptors can be internalized by clathrin-dependent and clathrin-independent, membrane-raft dependent mechanisms. TGF-β receptors internalized via clathrin-coated pit mediated endocytosis traffic to Smad anchor for receptor activation (SARA)-containing early endosome and propagate signal transduction. TGF-β receptors in the early endosome can be recycled back to the plasma membrane in a Rab11-dependent manner. TGF-β receptors internalized by membrane-raft dependent endocytosis traffic to caveolin-1 positive vesicles, where they are targeted for Smad7/Smurf2-mediated ubiquitination and proteosomal/lysosomal degradation. Other potential intracellular trafficking pathways for TGF-β receptors, including bi-directional trafficking between the early endosome and caveolin-1 positive vesicles (intermediate pathways) or direct trafficking of TGF-β receptors from the early endosome to proteosomal/lysosomal degradation pathways, are current topics of investigation. Smurf, Smad ubiquitination regulatory factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound