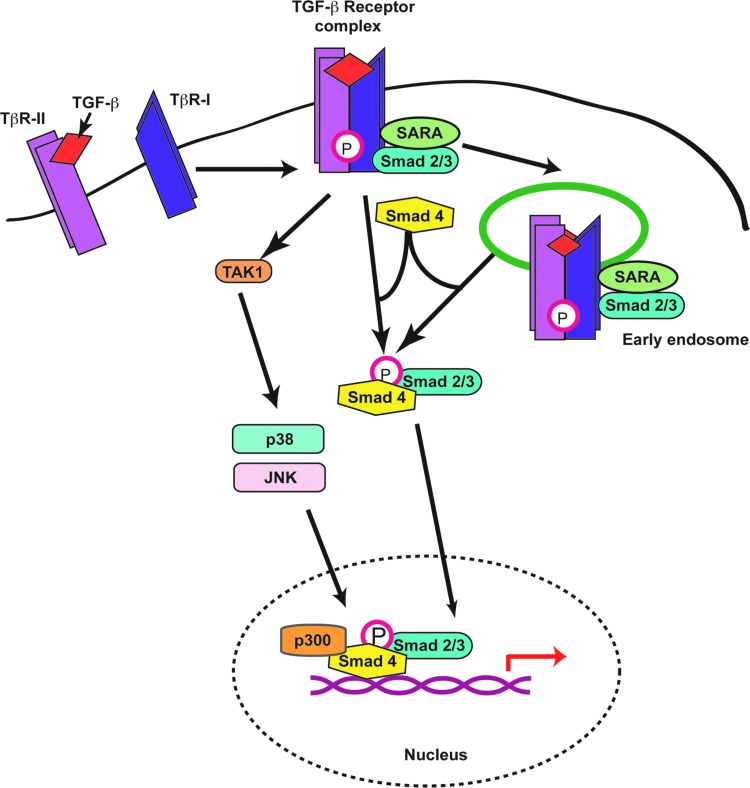
Figure 2.
TGF-β-signaling pathways. Binding of TGF-β to TβR-II induces association with and phosphorylation of TβR-I. The kinase properties of the latter are thus activated, which results in phosphorylation of Smad2 and/or Smad3 and their association with Smad4. Although this step occurs at the plasma membrane, active, ligand-bound TGF-β receptor complexes can also be endocytosed, thus triggering Smad2/3 phosphorylation from endosomal vesicles. The active phospho-Smad complexes then shuttle from the cytoplasm to the nucleus, where they associate with transcriptional cofactors, such as p300, to modulate transcription of target genes. TGF-β receptor complexes also initiate other noncanonical signaling pathways, including activation of the TAK1 kinase, which in turn induces phosphorylation of mitogen-activated kinases, such as p38 and JNK. Ultimately, the activation of these kinases also contributes to the transcriptional regulation of target genes. TAK1, TGF-β–activated kinase 1; TβR, TGF-β receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound