Abstract
Diabetes mellitus is a multifunctional disorder with several causes and multiple consequences. Nutraceuticals play a vital role in ameliorating diabetic condition. The stems of the plant, Tinospora cordifolia (T. cordifolia) are often used in Ayurvedic medicine for the management of diabetes. Earlier studies have shown that T. cordifolia to be a potent antidiabetic plant material by virtue of being rich in nutraceuticals. In the present study we were interested to know if, T. cordifolia stem extracts are able to promote glucose uptake through glucose transporters, 1 (GLUT1) and 3 (GLUT3), which are responsible for basal glucose uptake. Hence, Ehrlich ascites tumor (EAT) cells were chosen as a model which harbours both GLUT1 and GLUT3 and glucose uptake was measured using a fluorescent analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose (2-NBDG). Serially, solvent extracted T. cordifolia stems, especially water, ethanol and methanol extracts showed glucose uptake activity. Uptake was stimulated in a dose dependent manner at dosages of 1–100 μg. Glucose-stimulating activity does not seem to be solely due to polyphenol content since methanol extract, with high amount of polyphenol content (9.5 ± 0.1 g kg−1), did not stimulate higher glucose uptake activity when compared to water extract.
Keywords: Tinospora cordifolia, Glucose uptake, EAT cells, Diabetes
Diabetes mellitus is a chronic metabolic disorder which is characterized by hyperglycemia, relative insulin insufficiency and insulin resistance. Oral hypoglycemic drugs and dietary manipulation play a prominent role in the treatment of diabetes (Gray and Flatt 1997). Herbal and medicinal plants are used as alternative treatment modes for diabetes (Alarcon-Aguilara et al. 1998). In recent years, development of plant-based therapeutics is gaining ground, because of its perception of being safe. The anti-diabetic plant-based therapeutics could benefit the diabetic condition by promoting glucose uptake into tissues and/or enhancing insulin secretion from pancreatic β-cells (Gray and Flatt 1998). Glucose uptake by peripheral tissues is the most common mechanism by which, high glucose in the blood stream is reduced after a meal. Search for new anti-diabetic agents that can promote glucose uptake and enhance insulin secretion is one of the important aspects in diabetes research.
Tinospora cordifolia (Willd.) Miers ex Hook. f & Thoms (T. cordifolia) belongs to the Menispermaceae family and known as Gulancha in English. It is a large, glabrous, deciduous, climbing succulent shrub, commonly found in hedges. The plant stem has been considered as an indigenous source of medicine with anti-diabetic (Gupta et al. 1967), immunomodulatory (Atal et al. 1986), hepatoprotective (Peer and Sharma 1989) and anti-pyretic (Vedavathy and Rao 1991) actions. It is reported that the daily administration of either ethanol or aqueous extracts of T. cordifolia decreases the blood glucose level and increases glucose tolerance in rodents (Grover et al. 2001). T. cordifolia is widely used in Indian Ayurvedic medicine as a tonic, vitalizer and as a remedy for metabolic disorders (Chopra et al. 1982).
Nevertheless, antiglycemic action of T. cordifolia has not yet been fully elucidated. T. cordifolia aqueous extract was found to exhibit insulinotropic effect thereby significantly lowering blood glucose and increasing plasma insulin levels in alloxan-induced moderately diabetic rats (Noor et al. 1989; Noor and Ashcroft 1998). Although antihyperglycemic activity of T. cordifolia has been demonstrated in diabetic rats, its effect on glucose uptake is not known.
Glucose uptake in cells occurs by glucose transporters (GLUT). They are of various types. GLUT2 and GLUT4 transport glucose into cells in an insulin-dependent pathway. On the other hand GLUT1 and GLUT3 do not depend on insulin for translocation of glucose. They are the main transporters which are able to promote basal glucose uptake. Although fairly a good amount of work has been reported on the anti-diabetic property of T. cordifolia, mechanism of action in relation to its ability to transport glucose through GLUT transporters is seldom studied. Previously, we have demonstrated glucose uptake by banana flower and stem extracts (Jamuna et al. 2011). This study focuses on the ability of T. cordifolia extracts to transport glucose in EAT cells which use GLUT1 and GLUT3 primarily for basal glucose uptake.
Materials and methods
Plant material and preparation of extracts
T. cordifolia stems were collected from CFTRI campus, Mysore, Karnataka state, India. The plant was authenticated by depositing herbarium sheets at the Herbarium Collection Centre (SKU — accession no. 11199), Sri Krishnadevaraya University, Anantapur, India. T. cordifolia stems were cut into small pieces and dried in an oven at 40 °C. Then it was powdered and stored at 4 °C for further use. T. cordifolia stem powder was serially extracted with solvents viz., chloroform-ether (1:1), ethyl acetate, acetone, methanol, ethanol and water in that order. Extraction was done twice with each solvent (500 ml) and pooled. Solvents of individual extracts were flash evaporated to dryness, reconstituted with water, lyophilized and yields noted (Table 1). Of these, aqueous, ethanol and methanol extracts were used to study effect on glucose uptake and EAT cells were used as a model system.
Table 1.
Yield of different extracts of T. cordifolia stem powder (g/Kg−1 dry powder)
| Extracts | (g Kg−1dry powder) |
|---|---|
| Water | 68.0 ± 2.11 |
| Ethanol | 6.4 ± 1.01 |
| Methanol | 19.2 ± 2.09 |
| Acetone | 5.0 ± 0.84 |
| Ethyl Acetate | 7.0 ± 1.03 |
Values are expressed as mean ± SD of quadruplicates of two independent extractions
Preparation of EAT cells
EAT cells were maintained by injecting them (8 × 108 cells in 0.9% saline) intra-peritoneally in adult Swiss albino mice of 60 days old (Jamuna et al. 2011). The study had the clearance of Institutional Animal Ethical Committee. Mice were housed in polypropylene cages with sawdust as a bedding material and were fed with commercially available pellet diet ad libitum. Ascites fluid with EAT cells was collected by centrifugation at 1,000 g for 5 min from implanted adult Swiss albino mice after 10 days. Cell pellet containing EAT cells was washed twice with 4–5 volumes of 0.9% saline. Cell counting was done using haemocytometer by staining the cells with trypan blue. Cells were then used for glucose uptake studies in vitro.
Measurement of glucose uptake in EAT cells
Glucose uptake in EAT cells was measured by using 2-[N-(7- nitrobenz-2-oxa-1, 3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) as described earlier (Jamuna et al. 2011). Briefly, Krebs Ringer HEPES (KRH) buffer containing 136 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgCl2, 10 mM of N-2-hydroxyethyl piperazine N-2-ethane sulphonic acid (HEPES) salt (pH 7.4) was added to the pellet containing EAT cells (8 × 107). EAT cells were preincubated with different doses of extracts at 37 °C for 30 min. 2-NBDG (40 μl, 1 mM) was added to the cell suspension and was further incubated at 37 °C for 30 min. Excess 2-NBDG was removed by washing with KRH buffer. The cells were then lysed by suspending in KRH buffer containing 0.5% Triton-X-100 and sonicating for 10 min. Lyses of cells was ascertained by microscopic evaluation in the presence of trypan blue. After the lyses, they were centrifuged at 5,600 g and supernatant was used to measure 2-NBDG uptake using spectrofluorimeter with excitation and emission wavelengths set at 480 and 540 nm, respectively. Insulin which is a known promoter of glucose uptake was used as a positive control and was evaluated for its effect on glucose uptake in EAT cells by incubating with different amounts of insulin, instead of the extracts.
Analysis of polyphenol content
Total polyphenol content of T. cordifolia in different extracts was estimated using Folin-Ciocalteau reagent (Gao et al. 2000).
Statistical analyses
The experiments were repeated more than twice right from culturing the EAT cells. All values (average of 4 values) are given as net glucose uptake in nM which is obtained by subtracting values of control (EAT cells + 2-NBDG) from that of EAT cells + 2-NBDG + extracts. Data are presented as mean ± SD of quadruplicates of two independent experiments. Statistical analyses were done to determine the significance between values of different extracts by one-way analysis of variance (ANOVA) with post test followed by Tukey-Kramer multiple comparisons test using Instat statistical software. Levels of significance are indicated as follows, *P < 0.05, **P < 0.01 and ***P < 0.001.
Results and discussion
T. cordifolia stem (dried and powdered) serially extracted with various solvents, gave yields as depicted in Table 1. Among the extractants used, extraction with water gave the highest yield which was followed by methanol, ethanol, ethyl acetate and acetone. Total phenol content was determined in different extracts of T. cordifolia stem. Methanol extract of T. cordifolia showed high content of polyphenol, when compared to other extracts. Total polyphenol content in methanol extract of T. cordifolia was 9.5 ± 0.1 g Kg−1 extract, water extract was 2.1 ± 0.2 g Kg−1 and ethanol extract was 2.7 ± 0.1 g Kg−1.
Glucose uptake stimulatory activity of the lyophilized extracts of T. cordifolia viz; aqueous, ethanol and methanol were evaluated for glucose uptake at different dosages after reconstituting them in water. Among the extracts, ethyl acetate and acetone extracts could not be solubilised in water and hence were not taken up for further studies. All extracts exhibited little or no auto fluorescence when determined by spectrofluorimetry.
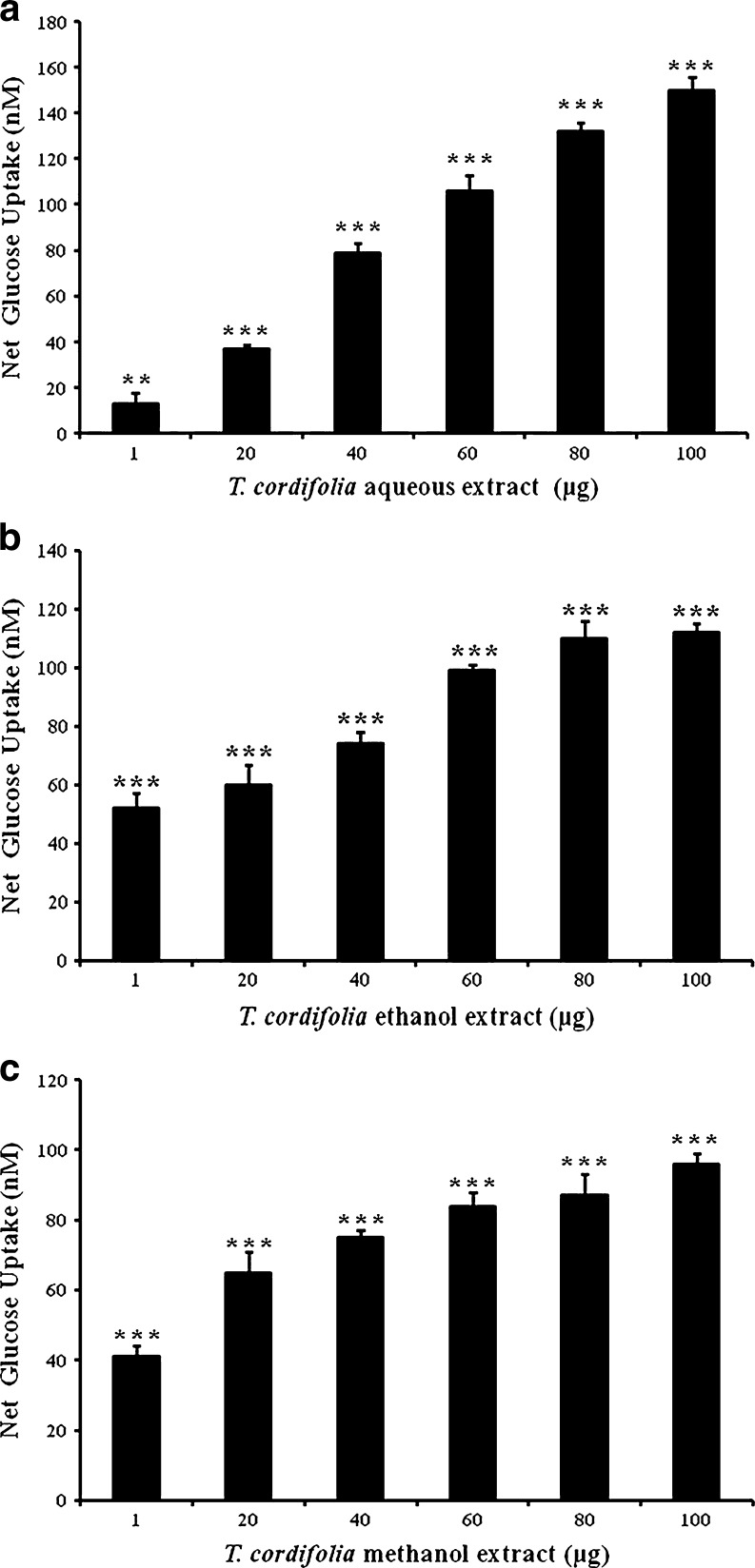
Aqueous extract of T. cordifolia showed potent glucose uptake activity, compared to other extracts at 100 μg. At dosages of 1–100 μg there was a gradual increase in glucose uptake (Fig. 1a). Ethanol extract of T. cordifolia showed increase in net glucose uptake from 1 to 100 μg which were 52.3, 60.1, 74.1, 99.0, 110.5 and 112.2 nM, respectively, when compared to control (Fig. 1b). At 1 μg, ethanol extract of T. cordifolia showed more potent activity in promoting glucose uptake when compared to other extracts. Methanol extract showed better glucose uptake, at lower dosage (40 μg), when compared to other extracts (Fig. 1c).
Fig. 1.
Effect of aqueous (a), ethanol (b) and methanol (c) extracts of T. cordifolia on glucose uptake in EAT cells: EAT cells (8 × 107) were incubated with different extracts of T. cordifolia, 40 μl of 1 mM 2-NBDG and volume made to 400 μl with KRH buffer (see Materials and methods). Uptake was measured after cell lyses using a spectrofluorimeter. Values are given as net glucose uptake in nM and are expressed as mean ± SD of quadruplicates of two independent experiments. Values are significant at ** P < 0.01 and *** P < 0.001 when compared to control (8 × 107 EAT cells + 40 μl of 1 mM 2-NBDG)
Glucose transport through biological membranes requires specific transport proteins. Passive transport (facilitated diffusion) of glucose through the cellular membrane is otherwise catalyzed by glucose carriers/transporters (protein symbol GLUT). GLUT 1 and GLUT 3 are the transmembrane carrier proteins of GLUT family. These are the most abundantly present glucose transporters in human tissues and are present in cell lines akin to EAT cells. These are responsible for basal glucose uptake and are the most prominent glucose transporter isoforms expressed in adult brain (Kayano et al. 1988; Nagamatsu et al. 1992).
T. cordifolia, a potent anti-diabetic herb has been widely used in Ayurvedic medicine. In this study, we could show that, three major extracts of T. cordifolia stem (aqueous, ethanol and methanol) were able to promote glucose uptake in EAT cells especially at lower doses. At higher doses, the uptake was inhibited. This could be either because of saturation levels attained by GLUT (Mueckler 1994) or by a feedback mechanism of the cell system. Reports on the glucose uptake activity by T. cordifolia have been few and far between. Aqueous extracts of T. cordifolia have been reported to enhance glucose transport activity in L6 myotubes in an insulin-dependent pathway in a time and dose dependent manner (Noipha et al. 2008). In another study, aqueous and ethanol extracts of T. cordifolia could ameliorate the metabolic derangements in lipid metabolism caused by streptozotocin in diabetic rats (Puranik et al. 2008). In yet another study, the methanol extract of T. cordifolia was found to have potent antidiabetic activity (Rajalakshmi et al. 2009). The elevated levels of glycosylated haemoglobin were reduced in diabetic rats treated with T. cordifolia methanol extract.
To correlate the glucose uptake to total phenolic acid content, each extract was analysed for total phenolic acid content. Methanol extract showed highest amount of phenolic acids quantitatively among the three. However, it did not translate into increased glucose uptake. It is conceivable that phenolic acids are not the only ones to stimulate glucose uptake. Apparently there may be other compounds which can promote glucose uptake. T. cordifolia has been found to contain alkaloids, diterpenoid lactones, glycosides, steroids, sesquiterpenoid and aliphatic compounds. Alkaloids like berberine, palmatine, tembetarine and magnoflorine have been isolated from the ethanol and dichloromethane extract of the stem (Singh et al. 2006). The major compound present in methanol extract of T. cordifolia is berberine (Panchabhai et al. 2008). Earlier berberine has been reported to activate AMP protein which exhibits beneficial metabolic effect in diabetic and insulin-resistant states (Lee et al. 2006). The insulin and c-peptide levels were considerably increased after treatment with the extracts of T. cordifolia, where methanol extract has given a better result (Rajalakshmi et al. 2009). Puranik et al. (2007) showed that diabetic rats treated with aqueous and ethanol extracts of T. cordifolia modulate renal tissue morphology and ameliorates the activity of key enzymes in gluconeogenic pathway. In another study (−) epicatechin was identified in methanol extract and showed antioxidant activity in various in vitro methods studied (Pushp et al. 2011).
In our earlier paper, we have shown that glucose uptake activity was promoted by extracts of banana flower and stem to various extents in EAT cells (Jamuna et al. 2011). The glucose promoting activity by T. cordifolia by GLUT 1 and GLUT 3 in EAT cells can have far reaching implications especially during diabetic condition. GLUT 1 and GLUT 3 are the major glucose transporters involved in basal glucose uptake, and the ameliorotary effect of T. cordifolia during diabetic condition could be through one such mechanism, apart from its insulin secretagogue effect.
Conclusion
In conclusion, this study clearly demonstrates for the first time that different extracts of T. cordifolia show stimulatory glucose uptake activity in EAT cells.
Acknowledgement
Mr. J. Darukeshwara thanks Indian Council of Medical Research (ICMR), New Delhi, for the award of Senior Research Fellowship.
References
- Alarcon-Aguilara FJ, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber CC, Flores-Saenz JL. Study of the anti-hyperglycemic effect of plants used as antidiabetic. J Ethnopharmacol. 1998;61:101–110. doi: 10.1016/S0378-8741(98)00020-8. [DOI] [PubMed] [Google Scholar]
- Atal CK, Sharma ML, Kaul A, Khajuria A. Immunomodulating agents of plant origin. I: preliminary screening. J Ethnopharmacol. 1986;18:133–141. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- Chopra RN, Chopra LC, Handa KD, Kapur LD. Editors: indigenous drugs of India. 2. Kolkota: M/S Dhar VN & sons; 1982. pp. 426–428. [Google Scholar]
- Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Gray AM, Flatt PR. Nature’s own pharmacy: the diabetes perspective. Proc Nutr Soc. 1997;56:507–517. doi: 10.1079/PNS19970051. [DOI] [PubMed] [Google Scholar]
- Gray AM, Flatt PR. Antihyperglycemic actions of Eucalyptus globules (Eucalyptus) are associated with pancreatic and extra-pancreatic effects in mice. J Nutr. 1998;128:2319–2323. doi: 10.1093/jn/128.12.2319. [DOI] [PubMed] [Google Scholar]
- Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76:233–238. doi: 10.1016/S0378-8741(01)00246-X. [DOI] [PubMed] [Google Scholar]
- Gupta SS, Verma SC, Garg VP, Rai M. Antidiabetic effects of Tinospora cordifolia. Part 1. Effect on fasting blood sugar level, glucose tolerance and adrenaline induced hyperglycaemia. Indian J Med Res. 1967;55:733–745. [PubMed] [Google Scholar]
- Jamuna JB, Salimath PV, Nandini CD. Stimulation of glucose uptake by Musa sp. (cv. elakki bale) flower and pseudostem extracts in Ehrlich ascites tumor cells. J Sci Food Agric. 2011;91:1482–1487. doi: 10.1002/jsfa.4337. [DOI] [PubMed] [Google Scholar]
- Kayano T, Fukumoto H, Eddy RL, Fan YS, Byers MG, Shows TN, Bell GI. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988;263:15245–15248. [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992;267:467–472. [PubMed] [Google Scholar]
- Noipha K, Purintrapiban J, Herunsalee A, Ratanachaiyavong S. In vitro glucose uptake activity of Tinospora crispa in skeletal muscle cells. Asian Biomedicine. 2008;2:415–420. [Google Scholar]
- Noor H, Ashcroft SJ. Pharmacological characterization of the antihyperglycemic properties of Tinospora crispa extract. J Ethnopharmacol. 1998;62:7–13. doi: 10.1016/S0378-8741(98)00008-7. [DOI] [PubMed] [Google Scholar]
- Noor H, Hammonds P, Sutton R, Ashcroft SJ. The hypoglycemic and insulinotropic activity of Tinospora crispa: studies with human and rat islets and HIT-T15 B cells. Diabetologia. 1989;32:354–359. doi: 10.1007/BF00277258. [DOI] [PubMed] [Google Scholar]
- Panchabhai TS, Kulkarni UP, Rege NN. Validation of therapeutic claims of Tinospora cordifolia: a review. Phytother Res. 2008;22:425–441. doi: 10.1002/ptr.2347. [DOI] [PubMed] [Google Scholar]
- Peer F, Sharma MC. Therapeutic evaluation of Tinospora cordifolia in CCl4 induced hepatopathy in goats. Indian J Veter Med. 1989;9:154–156. [Google Scholar]
- Puranik NK, Kammar KF, Sheela DR. Modulation of morphology and some gluconeogenic enzymes activity by Tinospora cordifolia (Willd.) in diabetic rat kidney. Biomed Res. 2007;18:179–183. [Google Scholar]
- Puranik NK, Kammar KF, Sheela DR. Efficacy of Tinospora cordifolia (Willd.) extracts on blood lipid profile in streptozotocin diabetic rats. Is it beneficial to the heart. Biomed Res. 2008;19:92–96. [Google Scholar]
- Pushp P, Sharma N, Joseph GS, Singh RP (2011) Antioxidant activity and detection of (−) epicatechin in the methanolic extract of stem of Tinospora cordifolia. J Food Sci Technol doi:10.1007/s13197-011-0354-8 [DOI] [PMC free article] [PubMed]
- Rajalakshmi M, Eliza J, Priya CE, Nirmala A, Daisy P. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats. Afr J Pharmacy Pharmacol. 2009;3:171–180. [Google Scholar]
- Singh RP, Banerjee S, Kumar PV, Raveesha KA, Rao AR. Tinospora cordifolia induces enzymes of carcinogen/drug metabolism and antioxidant system, and inhibits lipid peroxidation in mice. Phytomedicine. 2006;13:74–84. doi: 10.1016/j.phymed.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Vedavathy S, Rao KN. Antipyretic activity of six indigenous medicinal plants of Tirumala Hills Andhra Pradesh, India. J Ethnopharmacol. 1991;33:1–2. doi: 10.1016/0378-8741(91)90153-5. [DOI] [PubMed] [Google Scholar]