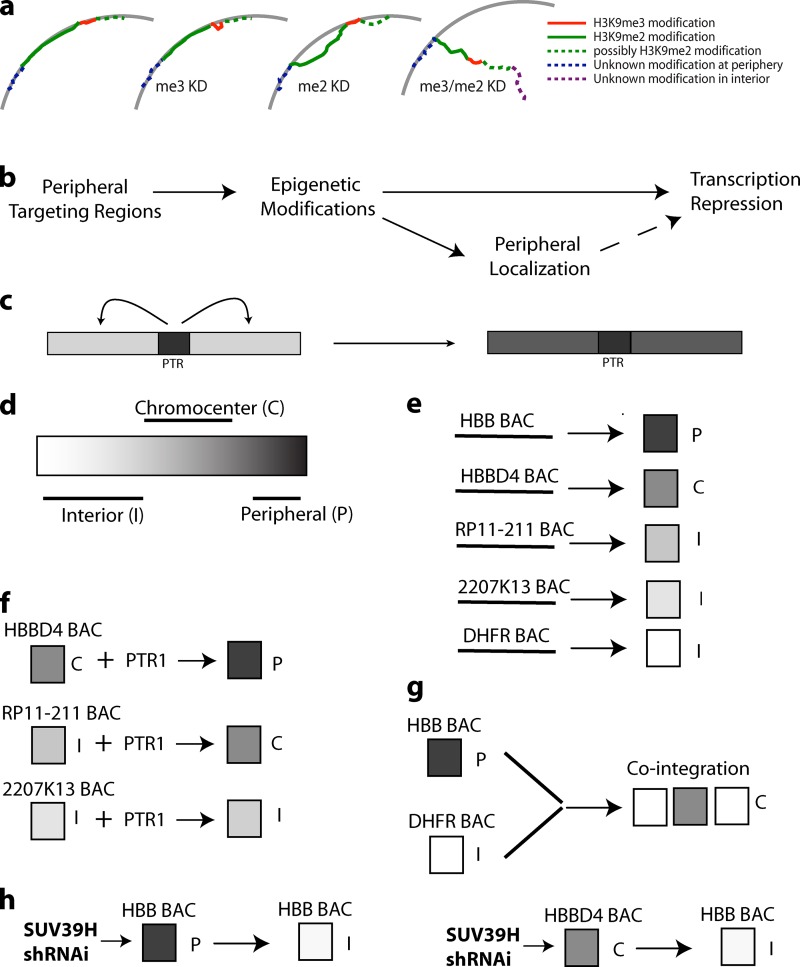
Figure 10.
Working model for HBB locus nuclear targeting. (a) At least two independent peripheral targeting mechanisms act on adjacent sequences to anchor the HBB locus and surrounding sequences to the periphery: a Suv39H/H3K9me3 (me3)-dependent mechanism mediated by PTR sequences near the HBB locus (red), a G9a/H3K9me2 (me2)-dependent mechanism mediated by sequences in left flanking LAD region (green; possibly also in right flanking LAD, dotted green), and a likely third, uncharacterized mechanism acting to tether distal LAD after combined Suv39H and G9a knockdown/inhibition (dotted blue). KD, knockdown. (b) Peripheral targeting regions (PTRs) induce epigenetic modifications, leading to inhibition of gene expression and, in a fraction of cells, association with the nuclear periphery. Peripheral association may in turn reinforce gene repression. (c) Nucleation of H3K9me3 by PTR and its propagation, presumably with other epigenetic marks, to flanking genomic regions. (d) Epigenetic modifications continuum depicted as a white to black gradient with targeting to the nuclear interior (I; white), chromocenter (C; gray), or periphery (P; black) dependent on position within this continuum. (e) Cis-elements establish epigenetic states characteristic for each BAC transgene, resulting in differential nuclear targeting. (f) Addition of PTR1 shifts this continuum toward the black, altering nuclear targeting. (g) Long-range influence of cis-elements within DHFR BAC transgene shifts the epigenetic state of cointegrated HBB BAC transgenes from black (peripheral) to gray (chromocenter). (h) Reducing H3K9me3 by Suv39H KD shifts the epigenetic state toward white.