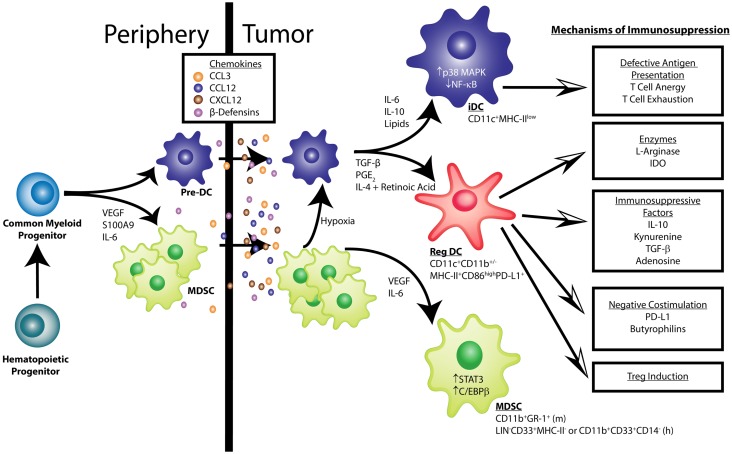
Figure 1.
Pathological dendritic cell differentiation contributes to tumor-induced immune evasion. Cancer-associated inflammation up-regulates the production of myeloid cells from hematopoietic progenitors in the bone marrow. Common myeloid progenitor cells give rise to pre-dendritic cells (pre-DCs) and, under the influence of tumor-derived factors (e.g., VEGF, IL-6, and S100A9), myeloid-derived suppressor cells (MDSCs). These myeloid cells migrate into the tumor microenvironment in response to chemokines such as CCL3, CCL12, CXCL12, and β-Defensins. VEGF and IL-6 activate STAT3 and C/EBPβ signaling in MDSCs, keeping them in an immature phenotype characterized as CD11b+Gr-1+in mice and LIN−CD33+MHC-II−or CD11b+CD33+CD14− in humans. Additionally, IL-6, IL-10, and the accumulation of lipids can activate p38 MAPK signaling to prevent the acquisition of classic DC function. Also, some MDSCs can differentiate into DCs in the hypoxic environment of the tumor. Immature DCs (iDCs) exhibit low NF-κB activation, express CD11c in mice and humans, and have low MHC-II levels. These iDCs are defective antigen presenters, which induces T cell anergy and exhaustion. In other conditions, like those found in epithelial ovarian cancer, pre-DCs mature into cells that express markers of conventional DCs (CD11c+ MHC-II+CD86high), but exert immunosuppressive functions, termed regulatory DCs (Reg DCs). Factors such as TGF-β, PGE2, IL-4, and retinoic acid have been shown to promote this altered maturation. These Reg DCs differ from conventional DCs in their ability to suppress effector T cell function through multiple mechanisms, which include: (1) secretion of the enzymes l-Arginase and IDO that result in the depletion of essential amino acids and production of the tolerogenic metabolites adenosine and kynurenine; (2) release of immunosuppressive factors such as IL-10 and TGF-β; (3) expression of costimulatory surface molecules, including PD-L1 and butyrophilins, that negatively regulate anti-tumor T cells; (4) induction of regulatory T cells (Tregs). More information regarding the source of the various secreted factors that govern the accumulation and function of tumor-associated myeloid cells can be found in recent reviews by Hanahan and Weinberg as well as Lindau et al. (17, 18).