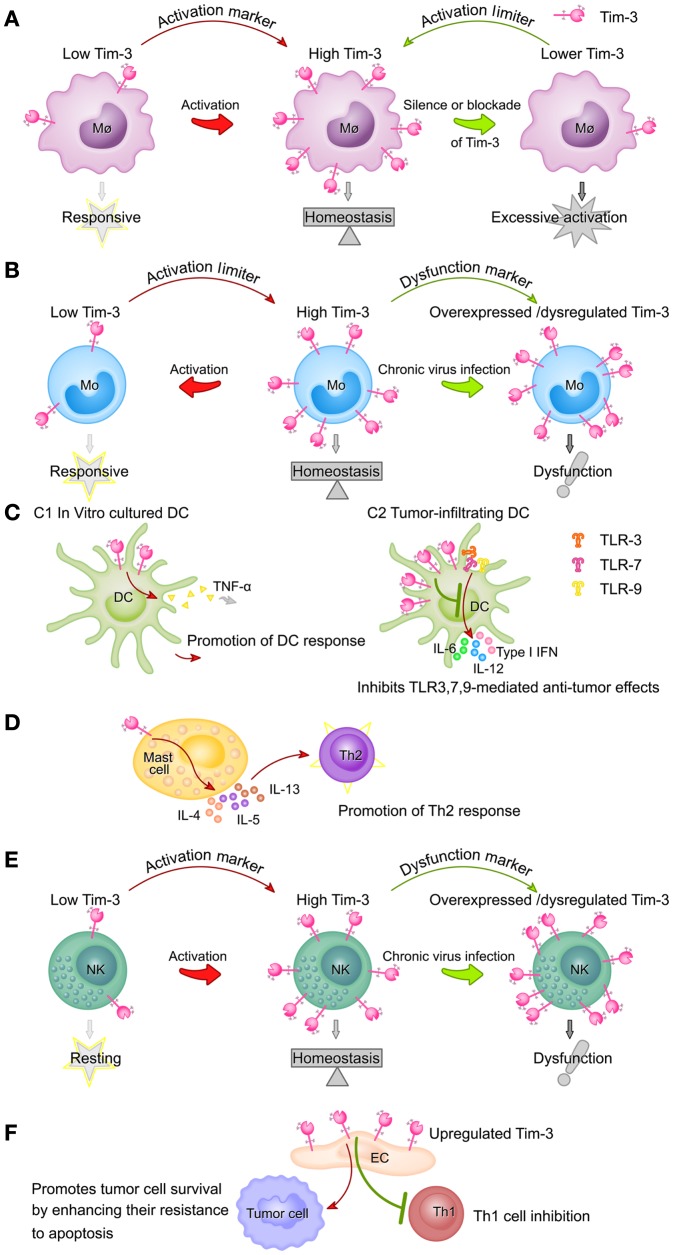
Figure 2.
Summary of Tim-3 expression on different innate immune cell populations and its functions. (A) Tim-3 acts as an activation marker of macrophages and a suppressor of macrophage activity. Tim-3 expression on macrophages is upregulated following activation (e.g., by LPS). High Tim-3 expression contributes to the homeostasis of macrophages, as blockade of the Tim-3 pathway leads to excessive macrophage activation. (B) Tim-3 acts as both an activation marker of monocytes and a suppressor of monocyte activity. Tim-3 is constitutively expressed on monocytes at high levels. Tim-3 expression decreases rapidly following stimulation of TLRs and this decrease correlates with increased monocyte activation. However, during chronic viral infection, Tim-3 is overexpressed on monocytes or cannot be downregulated, leading to the dysfunction or exhaustion of monocytes. (C) The roles of Tim-3 in dendritic cells are context-dependent. In in vitro cultured DCs, Tim-3 signaling promotes DC responses by increasing TNF-α production (C1), while, in tumor-infiltrating DCs, it inhibits the TLR3-, TLR7-, and TLR9-mediated anti-tumor effects of DCs (C2). (D) Tim-3 signaling in mast cells promotes Th2 responses by increasing IL-4, IL-5, and IL-13 production. (E) Expression of Tim-3 on NK cells is increased following activation and these increased Tim-3 levels act as a marker of fully mature and functional NK cells, but also potentially restrain NK cell function. During chronic viral infection, Tim-3 is overexpressed on NK cells or cannot be downregulated, both of which compromise NK cell function. (F) Tim-3 expression on the vascular endothelium is increased in the tumor microenvironment of lymphoma and melanoma, resulting in inhibition of Th1 cell function and increased tumor metastasis.