Obesity is considered among the top three leading causes of preventable death and illness in the United States (Danaei et al, 2009). In the United States and elsewhere, obesity's prevalence has risen considerably since the 1980s, with one-third of US adults now obese (http://www.win.niddk.nih.gov/statistics/). How health care approaches obesity is changing, with the American Medical Association recently defining obesity as a disease (http://www.bostonglobe.com/editorials/2013/06/28/ama-obesity-declaration-makes-third-america-ill/02nZ0a90RtlKE3hOWy59KK/story.html). Although the reasons why rates have risen are not entirely known and remain debated, the individual and societal costs necessitate an improved understanding. In this context, examining food and eating behaviors from interdisciplinary perspectives seems important in addressing an obesity epidemic.
Historically, obesity has been viewed from a metabolic perspective, with a focus on energy balance (Ziauddeen et al, 2012). More recently, it has been questioned whether obesity might be conceptualized within an addiction framework and whether certain foods may be addictive (Gearhardt et al, 2011a). Over time, a motivating factor for food consumption has shifted from sustenance and energy balance to pleasurable/hedonic purposes. Thus, motivational factors (positive-reinforcement-related anticipatory pleasure or negative-reinforcement-related stress reduction) might link to obesity similarly as in drug addictions. Additionally, metabolic factors implicated in homeostatic regulation may relate differently to these constructs in obese as compared with lean individuals.
To examine directly, we studied 25 obese and 25 matched lean individuals using a guided-imagery fMRI task that included individualized cues relating to personal stressors, favorite foods, or neutral-relaxing situations (Jastreboff et al, 2013). Obese as compared with lean individuals showed increased activation in cortico-striato-limbic structures (striatum, insula, inferior frontal gyrus and amygdala) to favorite-food cues, and activations of thalamus and striatum correlated with subjective craving in obese but not lean individuals. Similarly, stress-related activations in the striatum and insula correlated with food craving in obese but not lean individuals. Together, these findings suggest important differences in obese and lean individuals with respect to activation patterns in motivational neurocircuitry that may promote eating behaviors.
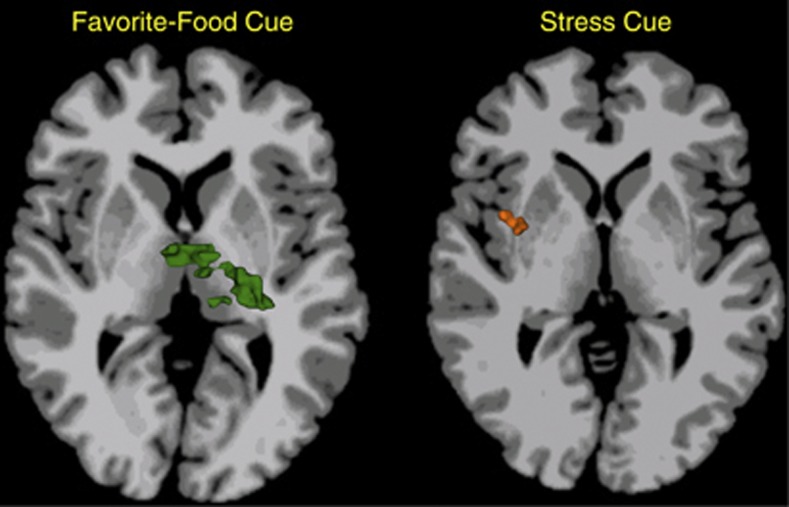
Importantly, metabolic measures were also collected. Brain activations during all three conditions correlated with a homeostatic measure of insulin resistance (HOMA-IR) in obese but not lean individuals in regions including the insula, inferior frontal gyrus, striatum, and thalamus. Furthermore, regional brain activations (eg, in the thalamus during the favorite-food cue condition and striatum and insula during the stress condition—Figure 1) were found to mediate the relationship between HOMA-IR and food craving in obese (but not lean) individuals. These findings suggest that interventions that target motivations rather than energy balance per se may be particularly relevant to combating obesity in the current environment.
Figure 1.
Overlaps in the relationships in obese individuals between brain activations and insulin resistance (HOMA-IR) and brain activations and food craving. During favorite-food cue exposure, individuals with obesity show thalamic activations that correlate both with HOMA-IR and food craving (left, green color). During stress cue exposure, individuals with obesity show insular and striatal (in putamen) activations that correlate both with HOMA-IR and food craving (right, orange color). Brain slices are located at Talaraich levels of z=6 (left) and z=4 (right), respectively. Right side of the brain is displayed on the left. Additional details of the original research can be found in Jastreboff et al (2013).
The current study helps integrate findings from multiple disciplines. Such integrative research may help address current debates about how best to conceptualize and treat obesity and ultimately lead to improved treatment strategies. Additionally, identifying clinically relevant subgroups with obesity (eg, those with binge-eating disorder, a condition hypothesized to show particular similarities with addictions (Gearhardt et al, 2011b), including in brain activations relating to reward processing (Balodis et al, 2013)), may help resolve current debates and target interventions.
FUNDING AND DISCLOSURE
Dr Potenza has consulted for Lundbeck and Ironwood pharmaceuticals; has had financial interests in Somaxon pharmaceuticals; received research support from Mohegan Sun Casino, Psyadon pharmaceuticals, the National Center for Responsible Gambling, the National Institutes of Health (NIH), Veterans Administration; has participated in surveys, mailings, or telephone consultations related to drug addiction, impulse-control disorders, or other health topics; has consulted for gambling, legal and governmental entities on issues related to addictions or impulse-control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has guest edited journal sections; has given academic lectures in grand rounds, Continuing Medical Education events, and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Acknowledgments
I would like to communicate gratitude and acknowledge the work of Cheryl Lacadie for generating the figure, and thank Ania Jastreboff, Rajita Sinha, Cheryl Lacadie, Dana Small, Robert Sherwin, and others for their work on the project investigating neural differences in obese and lean individuals as supported by RL1 AA017539. This research was funded in part by NIH grants from NIDA (P20 DA027844), NIAAA (RL1 AA017539), the Connecticut State Department of Mental Health and Addictions Services, and the Connecticut Mental Health Center. The funding agencies did not provide input or comment on the content of the manuscript, and the content of the manuscript reflects the contributions and thoughts of the author and not necessarily reflect the views of the funding agencies.
References
- Balodis IM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, et al. Monetary reward processing in obese individuals with and without binge eating disorder. Biol Psychiatry. 2013;73:877–886. doi: 10.1016/j.biopsych.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozzaffarian D, Taylor B, Rehm J, Murray CJL, et al. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011a;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, White MA, Potenza MN. Binge eating disorder and food addiction. Curr Drug Alcohol Rev. 2011b;4:201–207. doi: 10.2174/1874473711104030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food-cue-induced food craving in obesity: Association with insulin levels. Diabetes Care. 2013;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model. Nat Rev Neurosci. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]