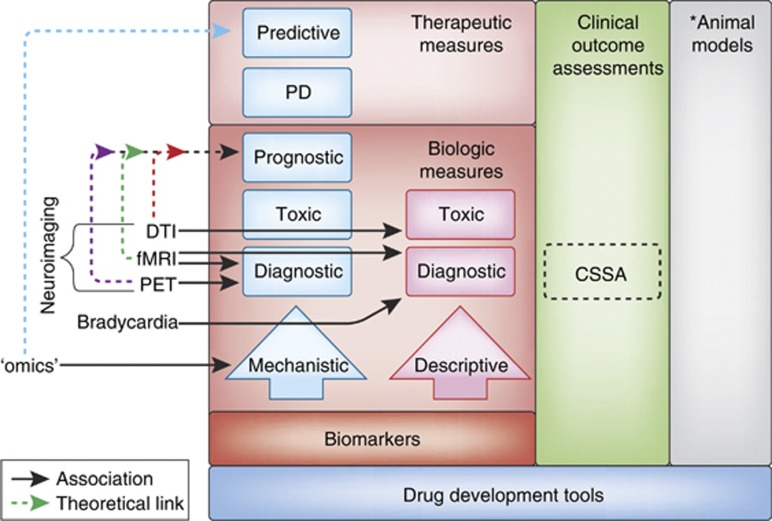
Figure 1.
Types of biomarkers. Biomarkers are presented hierarchically within the context of the FDA's set of Drug Development Tools (DDTs) (see ‘Introduction' for additional details). Biomarkers are classified into two primary tracts—‘descriptive' and ‘mechanistic'. Descriptive biomarkers are indirect, consequential correlates of the underlying pathophysiological processes. Mechanistic biomarkers represent a direct measure of the pathophysiological underpinnings of the disease process (see also ‘Future Directions'). Mechanistic biomarkers provide a foundation from which other types of biomarkers can be developed and are hence more ‘actionable'. Actionable biomarkers depicted have several possible clinical applications, and these types of biomarkers are hierarchically arranged. Predictive and pharmacodynamic (PD) biomarkers are measures of a therapeutic response (ie, light red box), whereas prognostic, toxic, and diagnostic markers are biological measures alone (ie, dark red box) (see also Text Box). As an example, a prognostic biomarker might provide a prodromal measure enabling physicians an opportunity to identify and medically manage individuals prior to the potential development of a substance use disorder (SUD). Otherwise, optimally, a predictive biomarker could help tailor the right drug to the right patient, at the right time by predicting disease progression (right patient), providing pharmacodynamic (PD) information to facilitate a therapeutic assessment of safety/efficacy (right drug), and predicting which individuals might respond to a particular therapy (right time). Ultimately, it is these biomarkers that are on the mechanistic pathway that will offer the greatest value for enhanced drug development and personalized medicine. Boxes in the left column refer to those methodologies as described within the text; solid lines refer to those approaches with some preliminary associations for a specific type of biomarker (see text); dotted lines refer to theoretical links across the various approaches (boxes). Within the context of the DDTs, the Cocaine Selective Severity Assessment (CSSA) is most appropriately described as an observer- or clinical-reported Clinical Outcome Assessment (COA) (see text). *Although they are one type of FDA-defined DDT, animal models (right column) are not discussed herein and this portion is therefore grayed out.