Summary
Although rare, synovial sarcoma (SS) is one of the most common soft tissue sarcomas affecting young adults. To investigate potential tumor markers related to synovial sarcoma prognosis, we carried out a single-institution retrospective analysis of 103 patients diagnosed with SS between 1980 and 2009. Clinical outcome data were obtained from medical records, and archived tissue samples were used to evaluate the relationship between progression-free survival (PFS) and several prognostic factors, including tumor expression of FGFR3 and FGFR4. No associations were found between PFS and gender, body mass index, tumor site, SS18-SSX translocation, or FGFR4 expression. As seen in previous studies, age at diagnosis (<35, 63% versus ≥35 years, 31% 10-year PFS; P = .033), histologic subtype (biphasic, 75% versus monophasic 34% 10-year PFS; P = .034), and tumor size (≤5 cm, 70% versus >5 cm, 22% 10-year PFS; P < .0001) were associated with PFS in SS patients. In addition, in a subset of patients with available archived tumor samples taken prior to chemotherapy or radiation (n = 34), higher FGFR3 expression was associated with improved PFS (P = .030). To the best of our knowledge, this is the largest study of SS to date to suggest a potential clinical role for FGFR3. While small numbers make this investigation somewhat exploratory, the findings merit future investigation on a larger scale.
Keywords: Local recurrence, Metastasis, Soft tissue sarcoma, Tissue microarray
1. Introduction
Synovial sarcoma (SS) is a rare but aggressive soft tissue sarcoma found most often in young adults. A US population-based study of patients with SS diagnosed between 1983 and 2005 found an overall five-year, cancer-specific survival rate of 80% in the pediatric group, but only 60% in adults [1]. Other studies show an even lower progression-free survival (PFS) among adults diagnosed with SS [2].
SS is molecularly associated with a t(X;18) translocation, resulting in a fusion protein combining SS18 with SSX1, SSX2, or rarely SSX4 [3–5]. While tumor resection margin, histologic grade, mitotic activity, histologic subtype, and SS18-SSX genotype have all been associated with outcome, the most consistent prognostic factors have been age at diagnosis and primary tumor size [6]. The FGFR4 Arg388Gly polymorphism associated with prolonged activation of the receptor [7], as well as FGFR4 RNA expression level and mutations in tumors, have been related to more aggressive disease and poor prognosis in a variety of soft tissue sarcomas [8–10]. FGFR3, best known for its role in regulating bone length, acts by inducing apoptosis and senescence in chondrocytes [11]. Ishibe et al found elevated expression of several fibroblast growth factor receptors, including FGFR3, FGFR4, and their ligands, in SS cell lines and tissues and also demonstrated that inhibiting these receptors in vitro, as well as in vivo, reduced SS growth [12]. Based on these prior studies, we evaluated the association of FGFR3 and FGFR4 protein expression and PFS in a population of patients with SS.
2. Materials and methods
2.1. Patients
The University of Minnesota Orthopedic Tumor Database was used to identify patients diagnosed with SS at the Fairview–University of Minnesota Medical Center between 1980 and 2009. This screening identified 103 patients. Deidentified patient data were extracted from medical records, including place of residence, gender, age and body mass index (BMI) at or near time of diagnosis, tumor site, tumor size, histological subtype, the presence of metastases at diagnosis, treatment, and follow-up through October 2010. Tumor size information was obtained from computed tomography or magnetic resonance imaging scans, if available, and from ultrasound or physical examination size estimates if not. A histological subtype had been assigned in most cases but was “not specified” or classified as “pleomorphic” in 17 tumors. The primary tumor site was noted as upper extremity, lower extremity, or trunk (which included extremity girdles such as hip, shoulder, and axilla). Available pathologic slides (n = 51) were reviewed by a single pathologist with experience in soft tissue tumors (J.C.M.) to confirm the diagnosis. If we were unable to obtain a histologic subtype diagnosis from medical records or if the diagnosis was disputed, the newly reviewed diagnosis was used for analyses.
2.2. SS18-SSX1 and SS18-SSX2 PCR
To evaluate the presence of SS18-SSX1 and SS18-SSX2 in patients with available archived tumor tissue, RNA was isolated from 53 formalin-fixed, paraffin-embedded (FFPE) SS tissue blocks using the Ambion FFPE RNA isolation kit (Ambion, Austin, TX). Total RNA was converted to cDNA using SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Real-time reverse transcriptase polymerase chain reaction was performed on cDNA using TaqMan primers and probes (Applied Biosystems, Foster City, CA) specific for SS18-SSX1 (Hs03024820_ft) and SS18-SSX2 (Hs03024398_ft) for 40 cycles, and products were verified by gel electrophoresis. Additionally, reverse transcriptase polymerase chain reaction was carried out using primers previously designed and used for this application [13]. All SS18-SSX fusion transcripts were amplified and then processed with XMNI designed to specifically digest the SS18-SSX2 fusion. Polymerase chain reaction products were then sequenced in the University of Minnesota’s Biomedical Genomics Center to confirm results. If SS18-SSX status was either negative or ambiguous following these methods, samples were assessed by Mayo Clinic’s anatomic molecular pathology lab using their standardized protocol for detecting SS18-SSX fusion transcripts. Samples reported as SSX1 or SSX2 had to agree by at least 2 of the methods above to be included in analyses (n = 40).
2.3. Tissue microarray construction and immunohistochemistry
Representative areas of SS with high tumor cell density were identified on hematoxylin and eosin–stained sections for 53 FFPE SS specimens. Tissue microarray (TMA) blocks consisting of quadruplicate 1.0 mm core samples were constructed with a manual tissue arrayer (MTA-1, Beecher Inc, Sun Prairie, WI) and limited to 64 cores per recipient block. Two additional cores were obtained when possible for RNA studies as described above.
Unstained TMA sections (4 μm) were deparaffinized and rehydrated using standard methods. For antigen detection and identification, slides were incubated in 6.0 pH buffer (Reveal Decloaking reagent, Biocare Medical, Concord, CA) in a steamer for 30 minutes at 95 to 98°C, followed by a 20-minute cool-down period. Slides were rinsed in running tap water, followed by immersion in Tris-buffered saline (TBS; pH 7.4). Subsequent steps were automated using an immunohistochemical (IHC) staining platform (Nemesis, Biocare). Endogenous peroxidase activity was quenched by slide immersion in 3% hydrogen peroxide solution (Peroxidazed, Biocare) for 10 min followed by TBS rinse. A serum-free blocking solution (Background Sniper, Biocare) was placed on sections for 10 minutes. Blocking solution was removed, and slides were incubated in primary antibody diluted in 10% blocking solution/90% TBS for 30 minutes at room temperature: rabbit anti-human FGFR3 polyclonal antibody (Abcam, Cambridge, MA; 1:400); rabbit anti-human FGFR4 polyclonal antibody (Abcam; 1:400); mouse anti-human vimentin monoclonal antibody (clone Vim3B4, Ventana Medical Systems, Inc, Tucson, AZ). Following incubation, Novocastra Post-Primary Block (Leica Microsystems Inc, Buffalo Grove, IL) was applied for 30 minutes at room temperature and rinsed with TBS. Novolink Polymer (Leica) was then applied for 30 min at room temperature and rinsed with TBS. Diaminobenzidine (Covance, Dedham, MD) was applied for 5 min followed by TBS rinse and counterstained with CAT Hematoxylin (Biocare) for 5 minutes. Slides were then dehydrated and protected with cover slip.
2.4. Slide digitization, annotation, and IHC quantification
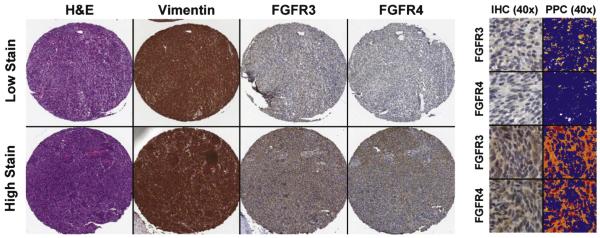
Digital images of IHC-stained TMA slides were obtained at 40× magnification (0.0625 μm2 per raw image pixel) using a whole slide scanner (ScanScope CS, Aperio Technologies, Vista, CA) fitted with a 40×/0.75 Plan Apo objective lens (Olympus, Center Valley, PA). Images were saved in SVS format (Aperio) on a server equipped with server software (ImageServer, Aperio) and retrieved using file management software (Spectrum, Aperio). Eight square regions (10 000 μm2 each) of representative tumor were annotated for each spot on the array. Immunohistochemical staining intensity within these regions was evaluated using Positive Pixel Count (v9, Aperio) image analysis software. Positive pixels (NTotal) in the brown (diaminobenzidine) colorimetric channel (intensity: 0-255) were further stratified into weak (NWP), moderate (NMP), and strong intensities (NSP) using upper-limit transmitted light intensity thresholds of 220, 175, and 100, respectively; where 255 is white and 0 is black. A metric of pixel intensity, IHC score, was defined as [(1*NWP) + (2*NMP) + (3*NSP)]/NTotal*100 and averaged across all samples for each patient separately for FGFR3 and FGFR4. Positive vimentin staining was used as a control for viable tissue; regions of negative staining were excluded from image analysis (Fig. 1). Cores from one patient were excluded from final analysis because they were obtained from a metastasis two years after primary diagnosis, rather than a primary tumor.
Fig. 1.
Positive pixel count analysis of FGFR3 and FGFR4 with vimentin(+) regions. SS TMA spots show representative low and high staining patterns for FGFR3 and FGFR4 (images at 10× magnification). High magnification images (40×) on the right display IHC staining and the correlating image analysis markup. Pseudocolors represent each IHC staining intensity range: blue, no staining; yellow, weak staining; orange, medium staining; and red, strong staining.
2.5. Statistical methods
Patient demographics and clinical characteristics were summarized for all patients and those with adequate samples to determine SS18-SSX translocation. Comparisons were made between groups using χ2 or Fisher exact tests, as appropriate. PFS was calculated from the date of diagnosis to the date of first known disease progression, relapse, or death, or was censored at the date of last contact to a maximum of 10 years for patients still alive without progressive disease. Variables considered for prediction of PFS included age at diagnosis (≤35 years, >35 years), gender, BMI (normal = <25, overweight = 25–29.9, obese = ≥30), tumor site (trunk, upper extremity, lower extremity), tumor size (≤5 cm, >5 cm), histology subtype (monophasic, biphasic), surgery type and treatment received, and FGFR3 and FGFR4 IHC score. Kaplan-Meier estimates and 95% confidence intervals (CI) for 5- and 10-year PFS were reported, and comparisons between groups were made using log-rank tests.
3. Results
A total of 103 patients were identified as having been diagnosed with SS in a 30-year period between 1980 and 2009 (patient characteristics summarized in the Table). The criteria for translocation status were met by 40 patients, with 60% having the SS18-SSX1 translocation and 40% with the SS18-SSX2 translocation. Of the characteristics assessed in this population, only gender and histological subtype had borderline relationships with translocation. SS18-SSX1 was more prevalent in men (P = .07) and equally distributed between subtypes. SS18-SSX2 was present in a higher percentage of monophasic SS samples (P = .10, Table 1).
Table.
Characteristics of sarcoma patients diagnosed from 1980 to 2009, including subset with samples reporting either SS18-SSX1 or SS18-SSX2 translocation
| All patients diagnosed (n = 103) |
Patient with samples reporting SS18 |
||||
|---|---|---|---|---|---|
| Total (n = 40) | SSX1 (n = 24, 60%) | SSX2 (n = 16 (40%) | P a | ||
| Variable | n (%) | ||||
| Age at diagnosis | .24 | ||||
| ≤35 y | 54 (52%) | 17 (43%) | 12 (50%) | 5 (31%) | |
| >35 y | 49 (48%) | 23 (58%) | 12 (50%) | 11 (69%) | |
| Sex | .07 | ||||
| Male | 59 (57%) | 22 (55%) | 16 (67%) | 6 (38%) | |
| Female | 44 (43%) | 18 (45%) | 8 (33%) | 10 (63%) | |
| BMI | .97 | ||||
| Normal (<25) | 35 (34%) | 11 (28%) | 6 (25%) | 3 (19%) | |
| Overweight (25-29.9) | 31 (30%) | 16 (40%) | 10 (42%) | 5 (31%) | |
| Obese (≥30) | 14 (14%) | 5 (13%) | 3 (13%) | 6 (38%) | |
| Unknown | 23 (22%) | 8 (20%) | 5 (21%) | 2 (13%) | |
| Site of tumor | .48 | ||||
| Trunk/non-extremity | 17 (17%) | 7 (18%) | 5 (21%) | 2 (13%) | |
| Upper extremity | 18 (18%) | 5 (13%) | 4 (17%) | 1 (6%) | |
| Lower extremity | 68 (66%) | 28 (70%) | 15 (63%) | 13 (81%) | |
| Tumor size, 5 cm cut point | .65 | ||||
| ≤5 cm | 40 (39%) | 12 (30%) | 6 (25%) | 6 (38%) | |
| >5 cm | 49 (48%) | 26 (65%) | 17 (71%) | 9 (56%) | |
| Unknown | 14 (14%) | 2 (5%) | 1 (4%) | 1 (6%) | |
| Tumor size, 7 cm cut point | .67 | ||||
| ≤7 cm | 51 (50%) | 22 (55%) | 12 (50%) | 10 (63%) | |
| >7 cm | 38 (37%) | 16 (40%) | 11 (46%) | 5 (31%) | |
| Unknown | 14 (14%) | 2 (5%) | 1 (4%) | 1 (6%) | |
| Histological subtype | .1 | ||||
| Monophasic | 53 (52%) | 23 (58%) | 11 (46) | 12 (75%) | |
| Biphasic | 33 (32%) | 17 (43%) | 13 (54%) | 4 (25%) | |
| Not specified | 17 (17%) | ||||
| Metastatic disease at diagnosis | 1.0 | ||||
| Yes | 6 (6%) | 2 (5%) | 1 (4%) | 1 (6%) | |
| No | 97 (94%) | 38 (95%) | 23 (96%) | 15 (94%) | |
| Surgery | |||||
| No surgery | 2 (2%) | ||||
| Marginal excision | 1 (1%) | ||||
| Excision/resection | 81 (79%) | ||||
| Amputation | 15 (15%) | ||||
| Unknown | 4 (4%) | ||||
| Radiation | |||||
| Yes | 69 (67%) | ||||
| No | 28 (27%) | ||||
| Unknown | 6 (6%) | ||||
| Chemotherapy | |||||
| Yes | 37 (36%) | ||||
| No | 63 (61%) | ||||
| Unknown | 3 (3%) | ||||
| Tumor free post-surgery | |||||
| No | 11 (11%) | ||||
| Yes | 80 (78%) | ||||
| Unknown | 12 (12%) | ||||
| Progression | |||||
| No | 62 (60%) | ||||
| Local recurrence | 9 (9%) | ||||
| Local relapse | 3 (3%) | ||||
| Distant recurrence | 26 (25%) | ||||
| Missing | 3 (3%) | ||||
NOTE. Percentages may not total 100% due to rounding.
χ2 or Fisher exact test as appropriate.
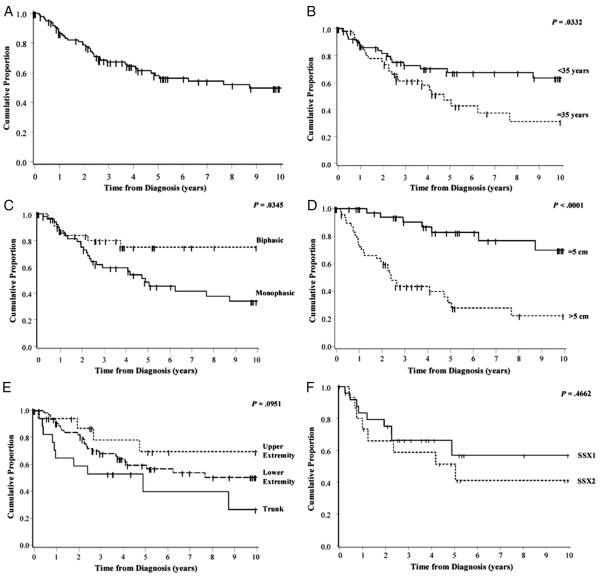
In this population, the 5- and 10-year PFS estimates were 58% (95% CI: 47%-68%) and 50% (95% CI: 37%-61%), respectively (Fig. 2A). As expected, those patients diagnosed at 35 or older had significantly inferior PFS than those diagnosed before age 35, with 10-year PFS of 31% (95% CI: 14%-50%) and 63% (95% CI: 47%-76%), respectively (P = .03, Fig. 2B). Gender and BMI at time of diagnosis (Supplementary Figure 1) did not appear to be statistically significantly associated with PFS. Histologic subtype analysis revealed that patients with the monophasic subtype had inferior PFS than those diagnosed with the biphasic subtype (P = .03, Fig. 2C), with 34% (95% CI: 19%-50%) 10-year PFS for monophasic and 75% (95% CI: 54%-87%) PFS for biphasic. Additionally, patients with tumors that were >5 cm in greatest diameter had significantly inferior PFS than those with tumors measuring 5 cm or less, with 10-year PFS estimates of 22% (95% CI: 9%-39%) and 70% (95% CI: 45%-85%), respectively (P < .0001, Fig. 2D). Although there was a slight trend in location of primary tumors with PFS (upper extremity > lower extremity > trunk), the overall differences between these groups were not statistically significant (P = .10, Fig. 2E). In the 40 patients with translocation information available, there was no significant difference in PFS based on translocation type (SS18-SSX1 versus SS18-SSX2, Fig. 2F).
Fig. 2.
Progression-free survival for SS patients diagnosed from 1980 to 2009. A, Ten-year PFS curves for all SS patients. B, By age at diagnosis (n = 103). C, By histological subtype, excluding 17 patients with unspecified or pleomorphic subtype (n = 86). D, By tumor size: >5 cm versus ≤5 cm, excluding 14 unknown. E, By tumor site: upper extremity, lower extremity, or trunk (n = 103). F, By translocation SS18-SSX1 versus SS18-SSX2 (n = 40) with cores available and quality RNA for detection of translocation.
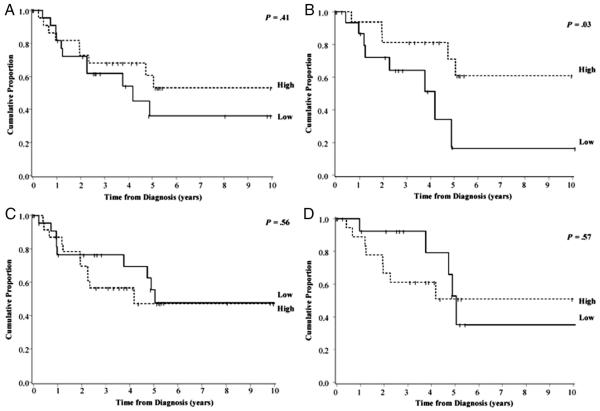
A total of 47 patients had sufficient tissue available to calculate IHC scores based on FGFR3 and FGFR4 expression. Neither FGFR3 nor FGFR4 expression in tumors was statistically significantly related to any of the demographic or clinical factors we assessed (Supplementary Table 1). Using the median IHC score to divide tumors into FGFR3 and FGFR4 high- and low-expression groups, we found no significant association between high versus low FGFR3 or FGFR4 expression and PFS when all cores passing quality check were included (Fig. 3A and 3C). However, only a subset (n = 34) of these tumor samples were taken from tumors resected prior to any chemotherapy or radiation treatment. In this subset, PFS in patients with primary tumors that expressed high levels of FGFR3 was significantly higher than those with lower IHC scores, with 61% (95% CI: 28%-82%) versus 17% (1%-51%) 10-year PFS, respectively (P = .03; Fig. 3B). FGFR4 expression in the tumor was not significantly associated with PFS, even after excluding cores of patients with prior exposure to chemotherapy and/or radiation (Fig. 3D).
Fig. 3.
Progression-free survival for SS patients diagnosed from 1980 to 2009 by levels of FGFR3 and FGFR4 expression in the tumor. Ten-year PFS curves for 47 SS patients based on high and low FGFR3 (A and B) and FGFR4 (C and D) expression. PFS was also examined after exclusion of tumors with prior exposure to radiation or chemotherapy (B and D).
4. Discussion
After evaluating demographic and clinical variables with outcome data from 103 patients diagnosed with SS at the Fairview University of Minnesota Medical Center between 1980 and 2009, we were able to address whether PFS in this patient population differed based on demographic and clinical characteristics found to be associated with survival in other studies of SS. Our results for age and tumor size were in agreement with a number of previous studies that found an association between inferior survival and older age at diagnosis [1,14–20] as well as primary tumor size >5 cm in largest diameter [1,2,14,15,20–27]. There was no evidence of an association with gender and PFS, and although we are the first to our knowledge to assess an association with BMI at diagnosis and PFS, we did not find a significant relationship.
Consistent with one prior study [21], we also found a significant association between monophasic subtype and inferior PFS. However, most of the studies to date have found either marginal or no association with monophasic or biphasic histologic subtype and outcome [1,14,16,18–20,22–26,28–30,32]. In our study, we also found that histological subtype was not significantly associated with fusion type. Other studies that carried out this comparison reported variable results; one prior study found no relationship between fusion and monophasic/biphasic subtype [31], two found marginally significant associations [29,30], while several other studies did find associations between these two factors [2,18,20,32]. In our study, we did not find an association between translocation variant and PFS; prior reports have had variable results for association of translocation with disease outcome [2,14,18,20,22,30–33].
A recent study demonstrated that transducing mouse myoblast cell lines with SS18-SSX2 resulted in elevated FGFR3 message compared to empty vector controls [27]. We detected FGFR3 and FGFR4 protein expression in a number of our SS tumor samples but found that this expression was not related to histological subtype or SS18-SSX translocation. These findings validate prior findings of FGFR3 and FGFR4 transcript expression and FGFR3 protein expression in SS frozen specimens and cell lines compared to other tumor types [12], although the previous study did not quantify differences in expression between SS18-SSX1– and SS18-SSX2–positive tumors. We did not find a significant difference in FGFR3 expression between monophasic and biphasic subtypes, concordant to the one prior study that assessed this relationship [12].
To our knowledge, this is the largest study that explores FGFR3 and FGFR4 protein expression in SS and its association with PFS. In our study, when post-treatment tumors were excluded, FGFR3 expression was associated with superior PFS. This is not the only malignancy to have improved prognosis associated with FGFR3 expression. In cervical cancer, one group found a general trend (albeit not significant) with improved disease-specific survival of patients with tumors expressing higher levels of FGFR3b mRNA [34]. In another study of patients with bladder cancer, while FGFR3 expression was not associated with survival, it was associated with earlier stage and lower-grade tumors [35]. The less prognostically dire phenotypes of these cancers might be explained by the inhibitory functions of FGFR3, as seen in prior studies where signaling through FGFR3 was shown to be important for inhibiting proliferation in developing chondrocytes [36,37].
FGFR4 appears to be important for growth and progression in another soft tissue sarcoma, pediatric rhabdomyosarcoma [9]. FGFR4 is not only elevated in rhabdomyosarcoma compared to normal muscle tissue [38], but the more aggressive PAX-FKHR-positive alveolar rhabdomyosarcoma subtype also appears to have elevated FGFR4 message compared to fusion-negative rhabdomyosarcoma [39]. Additionally, one study found FGFR4 expression to be related to poor prognosis in humans and development of metastases in mice [9] and the FGFR4 Gly388Arg polymorphism, has been associated with poor prognosis in combined sarcoma patients [8]. In our study, we did not find an obvious association between FGFR4 expression and PFS in SS patients, although we cannot rule out lack of power to detect an association due to small sample size. It should be noted that in the present study, on average there were many fewer cells staining positive (including weak, moderate, and strong staining) for FGFR4 (8.2%) than FGFR3 (49.2%). FGFR3 mRNA expression has been more consistently observed in SS tumors and cell lines than FGFR4 [12], and FGFR4 may therefore be less important in SS pathogenesis.
This study had several limitations. SS is a rare cancer, limiting sample size from a single institution. Patients also received diverse treatments (over the span of 30 years), and there may have been other unknown variables that influenced survival. The small sample size limited the ability to adjust for confounding factors. The effects of confounding and limited sample size might have restricted the ability to detect PFS differences according to FGFR3 or FGFR4 status for the whole population studied. We also used median cut-points of IHC data for analysis, but these may not be the biologically relevant cut-points for these receptors. This was a retrospective study relying on medical records at the University of Minnesota Medical Center for almost all of the collected information. Given the 30-year time period assessed, some information was not available for all patients, with many having incomplete follow-up. The analysis was also restricted to samples with available paraffin blocks to assess specific SS18-SSX translocation and FGFR3 and FGFR4 protein expression; it was further limited to samples with sufficient quality RNA to assess translocation.
In conclusion, our data support the previously identified prognostic factors in predicting PFS in SS, including histological subtype, tumor size, and age. Our study is the first to examine FGFR3 and FGFR4 tumor protein expression in relation to patient PFS. We found an association between elevated FGFR3 in SS tumors and improved PFS after excluding patients who had been treated with radiation or chemotherapy prior to excision of the tumor. This finding warrants further investigation in a larger sample of SS. Additionally, future investigations of mutations and splice variants in FGFR3 might also render findings that account for prognostic differences in SS patients.
Supplementary Material
Acknowledgments
This study utilized BioNet histology and digital imaging core facilities, which are supported by NIH grants P30 CA77598 (D. Yee), P50 CA101955 (D. Buchsbaum) and KL2 RR033182 (B. Blazar), and by the University of Minnesota Academic Health Center. The authors would also like to thank the Molecular Anatomic Pathology Laboratory members Barbara R. Evers, Christopher W. Roth, and Amber R. Seys for their work identifying fusion transcripts in the tumor samples.
Footnotes
Supplementary data Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.humpath.2013.03.001.
Grant Support: Supported by National Institutes of Health Pediatric Cancer Epidemiology Training Grant T32 CA099936 (B. Charbonneau); the Children’s Cancer Research Fund, Minneapolis, MN; and the Karen Wyckoff Rein in Sarcoma Foundation.
Disclosure/Conflict of Interest: There are no known conflicts of interest.
References
- [1].Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537–47. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- [2].Canter RJ, Qin LX, Maki RG, et al. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–7. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20:5755–62. doi: 10.1038/sj.onc.1204601. [DOI] [PubMed] [Google Scholar]
- [4].Skytting B, Nilsson G, Brodin B, et al. A novel fusion gene, SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst. 1999;91:974–5. doi: 10.1093/jnci/91.11.974. [DOI] [PubMed] [Google Scholar]
- [5].Mancuso T, Mezzelani A, Riva C, et al. Analysis of SYT-SSX fusion transcripts and bcl-2 expression and phosphorylation status in synovial sarcoma. Lab Invest. 2000;80:805–13. doi: 10.1038/labinvest.3780085. [DOI] [PubMed] [Google Scholar]
- [6].Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol. 2008;97:314–20. doi: 10.1002/jso.20974. [DOI] [PubMed] [Google Scholar]
- [7].Wang J, Yu W, Cai Y, Ren C, Ittmann MM. Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia. 2008;10:847–56. doi: 10.1593/neo.08450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morimoto Y, Ozaki T, Ouchida M, et al. Single nucleotide polymorphism in fibroblast growth factor receptor 4 at codon 388 is associated with prognosis in high-grade soft tissue sarcoma. Cancer. 2003;98:2245–50. doi: 10.1002/cncr.11778. [DOI] [PubMed] [Google Scholar]
- [9].Taylor JGVI, Cheuk AT, Tsang PS, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009;119:3395–407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao L, Yu Y, Bilke S, et al. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–508. doi: 10.1158/0008-5472.CAN-10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foldynova-Trantirkova S, Wilcox WR, Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum Mutat. 2012;33:29–41. doi: 10.1002/humu.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ishibe T, Nakayama T, Okamoto T, et al. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–12. doi: 10.1158/1078-0432.CCR-04-2057. [DOI] [PubMed] [Google Scholar]
- [13].Lasota J, Jasinski M, Debiec-Rychter M, et al. Detection of the SYT-SSX fusion transcripts in formaldehyde-fixed, paraffin-embedded tissue: a reverse transcription polymerase chain reaction amplification assay useful in the diagnosis of synovial sarcoma. Mod Pathol. 1998;11:626–33. [PubMed] [Google Scholar]
- [14].ten Heuvel SE, Hoekstra HJ, Bastiaannet E, Suurmeijer AJ. The classic prognostic factors tumor stage, tumor size, and tumor grade are the strongest predictors of outcome in synovial sarcoma: no role for SSX fusion type or ezrin expression. Appl Immunohistochem Mol Morphol. 2009;17:189–95. doi: 10.1097/PAI.0b013e31818a6f5c. [DOI] [PubMed] [Google Scholar]
- [15].Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999;85:2596–607. doi: 10.1002/(sici)1097-0142(19990615)85:12<2596::aid-cncr16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [16].Spillane AJ, A’Hern R, Judson IR, Fisher C, Thomas JM. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18:3794–803. doi: 10.1200/JCO.2000.18.22.3794. [DOI] [PubMed] [Google Scholar]
- [17].de Silva MV, McMahon AD, Reid R. Prognostic factors associated with local recurrence, metastases, and tumor-related death in patients with synovial sarcoma. Am J Clin Oncol. 2004;27:113–21. doi: 10.1097/01.coc.0000047129.97604.d6. [DOI] [PubMed] [Google Scholar]
- [18].Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol. 2004;22:4040–50. doi: 10.1200/JCO.2004.11.093. [DOI] [PubMed] [Google Scholar]
- [19].Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16:437–44. doi: 10.1093/annonc/mdi082. [DOI] [PubMed] [Google Scholar]
- [20].Sun Y, Sun B, Wang J, et al. Prognostic implication of SYT-SSX fusion type and clinicopathological parameters for tumor-related death, recurrence, and metastasis in synovial sarcoma. Cancer Sci. 2009;100:1018–25. doi: 10.1111/j.1349-7006.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115:2988–98. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]
- [22].Nilsson G, Skytting B, Xie Y, et al. The SYT-SSX1 variant of synovial sarcoma is associated with a high rate of tumor cell proliferation and poor clinical outcome. Cancer Res. 1999;59:3180–4. [PubMed] [Google Scholar]
- [23].Singer S, Baldini EH, Demetri GD, Fletcher JA, Corson JM. Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival. J Clin Oncol. 1996;14:1201–8. doi: 10.1200/JCO.1996.14.4.1201. [DOI] [PubMed] [Google Scholar]
- [24].Trassard M, Le Doussal V, Hacene K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–34. doi: 10.1200/JCO.2001.19.2.525. [DOI] [PubMed] [Google Scholar]
- [25].Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246:105–13. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Krieg AH, Hefti F, Speth BM, et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22:458–67. doi: 10.1093/annonc/mdq394. [DOI] [PubMed] [Google Scholar]
- [27].Garcia CB, Shaffer CM, Alfaro MP, et al. Reprogramming of mesenchymal stem cells by the synovial sarcoma-associated oncogene SYT-SSX2. Oncogene. 2012;31:2323–34. doi: 10.1038/onc.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lewis JJ, Antonescu CR, Leung DH, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087–94. doi: 10.1200/JCO.2000.18.10.2087. [DOI] [PubMed] [Google Scholar]
- [29].Mezzelani A, Mariani L, Tamborini E, et al. SYT-SSX fusion genes and prognosis in synovial sarcoma. Br J Cancer. 2001;85:1535–9. doi: 10.1054/bjoc.2001.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takenaka S, Ueda T, Naka N, et al. Prognostic implication of SYT-SSX fusion type in synovial sarcoma: a multi-institutional retrospective analysis in Japan. Oncol Rep. 2008;19:467–76. [PubMed] [Google Scholar]
- [31].Inagaki H, Nagasaka T, Otsuka T, et al. Association of SYT-SSX fusion types with proliferative activity and prognosis in synovial sarcoma. Mod Pathol. 2000;13:482–8. doi: 10.1038/modpathol.3880083. [DOI] [PubMed] [Google Scholar]
- [32].Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–60. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- [33].Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–40. [PubMed] [Google Scholar]
- [34].Rosty C, Aubriot MH, Cappellen D, et al. Clinical and biological characteristics of cervical neoplasias with FGFR3 mutation. Mol Cancer. 2005;4:15. doi: 10.1186/1476-4598-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bodoor K, Ghabkari A, Jaradat Z, et al. FGFR3 mutational status and protein expression in patients with bladder cancer in a Jordanian population. Cancer Epidemiol. 2010;34:724–32. doi: 10.1016/j.canep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- [36].Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–65. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- [37].Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- [38].Khan J, Wei JS, Ringner M, et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med. 2001;7:673–9. doi: 10.1038/89044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Davicioni E, Finckenstein FG, Shahbazian V, et al. Identification of a PAX-FKHR gene expression signature that defines molecular classes and determines the prognosis of alveolar rhabdomyosarcomas. Cancer Res. 2006;66:6936–46. doi: 10.1158/0008-5472.CAN-05-4578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.